Correlations between Volumetric Capnography and Automated Quantitative Computed Tomography Analysis in Patients with Severe COPD
Abstract
:1. Introduction
2. Methods
2.1. Pulmonary Function and Six-Minute Walk Test
2.2. High Resolution Computed Tomography
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Brown, R.H.; Brooker, A.; Wise, R.A.; Reynolds, C.; Loccioni, C.; Russo, A.; Risby, T.H. Forced Expiratory Capnography and Chronic Obstructive Pulmonary Disease (COPD). J. Breath Res. 2013, 7, 017108. [Google Scholar] [CrossRef] [Green Version]
- Wedzicha, J.Á.; Miravitlles, M.; Hurst, J.R.; Calverley, P.M.A.; Albert, R.K.; Anzueto, A.; Crimer, G.J.; Papi, A.; Rabe, K.F.; Rigau, D.; et al. Management of COPD exacerbations: A European Respiratory Society/American Thoracic Society guideline. Eur. Respir. J. 2017, 49, 1600791. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, P.G.; August, A.; Roche, N.; Singh, D.; Martinez, F.J. Current concepts in targeting chronic obstructive pulmonary disease pharmacotherapy: Making progress towards personalized management. Lancet 2015, 385, 1789–1798. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.H.; Hersh, C.P.; Castaldi, P.J.; Cho, M.H.; Raby, B.A.; Laird, N. Analyzing networks of phenotypes in complex diseases: Methodology and applications in COPD. BMC Syst. Biol. 2014, 8, 78. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, C.M. Some recent advances in the prevention and treatment of chronic bronchitis and related disorders with special reference to the effects of cigarette smoking. Proc. R. Soc. Med. 1965, 58, 918–928. [Google Scholar]
- Ingebrigtsen, T.; Thomsen, S.F.; Vestbo, J.; van der Sluis, S.; Kyvik, K.O.; Silverman, E.K.; Svartengren, M.; Backer, V. Genetic influences on chronic obstructive pulmonary disease—A twin study. Respir Med. 2010, 104, 1890–1895. [Google Scholar] [CrossRef]
- McCloskey, S.C.; Patel, B.D.; Hinchliffe, S.J.; Reid, E.D.; Wareham, N.J.; Lomas, D.A. Siblings of patients with severe chronic obstructive pulmonary disease have a significant risk of airflow obstruction. Am. J. Respir. Crit. 2001, 164, 1419–1424. [Google Scholar] [CrossRef]
- Silverman, E.K.; Chapman, H.A.; Drazen, J.M.; Weiss, S.T.; Rosner, B.; Campbell, E.J.; O’Donnel, W.J.; Reilly, J.J.; Ginns, L.; Mentzer, S.; et al. Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease. Risk to relatives for airflow obstruction and chronic bronchitis. Am. J. Respir. Crit. Care Med. 1998, 157, 1770–1778. [Google Scholar] [CrossRef]
- Zhou, J.J.; Cho, M.H.; Castaldi, P.J.; Hersh, C.P.; Silverman, E.K.; Laird, N.M. Heritability of chronic obstructive pulmonary disease and related phenotypes in smokers. Am. J. Respir. Crit. Care Med. 2013, 188, 941–947. [Google Scholar] [CrossRef] [Green Version]
- Menezes, A.M.B.; Victora, C.G.; Perez-Padilla, R.; PLATINO Team. The Platino project: Methodology of a multicenter prevalence survey of chronic obstructive pulmonary disease in major Latin America cities. BMC Med. Res. Methodol. 2004, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, S.M.; Paschoal, I.A.; De Capitani, E.M.; Moreira, M.M.; Palhares, L.C.; Pereira, M.C. COPD phenotypes on computed tomography and its correlation with selected lung function variables in severe patients. Int. J. COPD 2016, 11, 503–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, S.M.D. Bronquiectasias Associadas a DPOC Grave: Características Clínicas, Funcionais e Estruturais (Bronchiectasis Associated with Severe COPD: Clinical, Functional and Structural Features). Ph.D. Thesis, Universidade Estadual de Campinas, Faculdade de Ciências Médicas, Campinas, São Paulo, 2020; 101p. [Google Scholar]
- Vogelmeier, C.; Augusti, A.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Criner, G.; Frith, P.; Halpin, D.M.G.; Han, M.; Varela, M.V.L.; et al. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (Updated 2021). Available online: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf (accessed on 6 March 2021).
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; Van Der Grinten, C.P.M.; Gustafsson, P.; Hankinson, J.; et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117, Erratum in Am. J. Respir. Crit. Care Med. 2016, 193, 1185. [Google Scholar] [CrossRef] [PubMed]
- Enright, P.L.; Sherrill, D.L. Reference equations for the six-minute walk in healthy adults. Am. J. Respir. Crit. Care Med. 1998, 158, 1384–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achenbach, T.; Weinheimer, O.; Buschsieweke, C.; Heussel, C.P.; Thelen, M.; Kauczor, H.U. Fully automatic detection and quantification of emphysema on thin section md-ct of the chest by a new and dedicated software. RoFo 2004, 176, 1409–141516. [Google Scholar] [CrossRef]
- Heussel, C.P.; Herth, F.J.F.; Kappes, J.; Hantusch, R.; Hartlieb, S.; Weinheimer, O.; Kauczor, H.U.; Eberhardt, R. Fully-automatic quantitative assessment of emphysema in computed tomography—comparison with pulmonary function testing and normal values. Eur. Radiol. 2009, 19, 2391–2402. [Google Scholar] [CrossRef]
- Celli, B.R.; Cote, C.; Marin, J.M.; Casanova, C.; Oca, M.M.; Mendez, R.A.; Plata, V.P.; Cabral, H.J. The Body Mass Index, Airflow Obstruction, Dyspnea, Exercise Performance (BODE) index in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Wielpütz, M.O.; Eichinger, M.; Weinheimer, O.; Ley, S.; Mall, M.A.; Wiebel, M.; Arved, B.; Hans-Ulrich, K.; Heussel, C.P.; Puderbach, M. Automatic airway analysis on multidetector computed tomography in cystic fibrosis. J. Thorac. Imaging. 2013, 38, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Ostridge, K.; AERIS Study Group; Williams, N.P.; Kim, V.; Harden, S.; Bourne, S.; Clarke, S.C.; Aris, E.; Mesia-Vela, S.; Devaster, J.-M.; et al. Relationship of CT-quantified emphysema, small airways disease and bronchial wall dimensions with physiological, inflammatory and infective measures in COPD. Respir Res. 2018, 19, 31. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, J.D.; McKenzie, A.S.; Zach, J.A.; Wilson, C.G.; Curran-Everett, D.; Stinson, D.S.; Newell, J.D., Jr.; Lynch, D.A. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. Am. J. Roentgenol. 2013, 201, W460–W470. [Google Scholar] [CrossRef] [Green Version]
- Veronez, L.; Moreira, M.M.; Soares, S.T.P.; Pereira, M.C.; Ribeiro, M.A.G.O.; Ribeiro, J.D.; Terzi, R.G.G.; Martins, L.C.; Paschoal, I.A. Volumetric capnography for the evaluation of pulmonary disease in adult patients with cystic fibrosis and noncystic fibrosis bronchiectasis. Lung 2010, 188, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Verscheure, S.; Massion, P.B.; Verschuren, F.; Damas, P.; Magder, S. Volumetric capnography: Lessons from the past and current clinical applications. Crit. Care 2016, 20, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanch, L.; Romero, P.V.; Lucangelo, U. Volumetric capnography in the machanically ventilated patient. Minerva Anestesiol. 2006, 72, 577–585. [Google Scholar] [PubMed]
- Suarez-Sipmann, F.; Bohm, S.H.; Tusman, G. Volumetric capnography: The time has come. Curr. Opin. Crit. Care 2014, 20, 333–339. [Google Scholar] [CrossRef]
- Moreira, M.; Veronez, L.; Pereira, M.C.; Silva, S.; De Capitani, E.M.; Paschoal, I.; Barcaui, L. Volumetric capnography for the evaluation of chronic airways diseases. Int. J. Chron. Obstruct. Pulmon. Dis. 2014, 9, 983–989. [Google Scholar] [CrossRef] [Green Version]
- Schwardt, J.D.; Neufeld, G.R.; Baumgardner, J.E.; Scherer, P.W. Noninvasive recovery of acinar anatomic information from CO2 expirograms. Ann. Biomed. Eng 1994, 22, 293–306. [Google Scholar] [CrossRef]
- Ribeiro, M.G.O.; Silva, M.T.N.; Ribeiro, J.D.; Moreira, M.M.; Almeida, C.C.B.; Almeida-Junior, A.A.; Ribeiro, A.F.; Pereira, M.C.; Hessel, G.; Paschoal, I.A. Volumetric capnography as a tool to detect early peripheric lung obstruction in cystic fibrosis patients. J. Pediatr. 2012, 88, 509–517. [Google Scholar] [CrossRef]
- De Jong, P.A.; Lindblad, A.; Rubin, L.; Hop, W.C.; de Jongste, J.C.; Brink, M.; Tiddens, H.A. Progression of lung disease on computed tomography and pulmonary function tests in children and adults with cystic fibrosis. Thorax 2006, 61, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Coxson, H.O. Quantitative computed tomography assessment of airway wall dimensions: Current status and potential applications for phenotyping chronic obstructive pulmonary disease. Proc. Am. Thorac Soc. 2008, 5, 940–945. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.S.; Jin, G.Y.; Li, Y.Z.; Lee, J.E.; Shin, H.S. CT Quantification of Lungs and Airways in Normal Korean Subjects. Korean J. Radiol. 2017, 18, 739–748. [Google Scholar] [CrossRef]
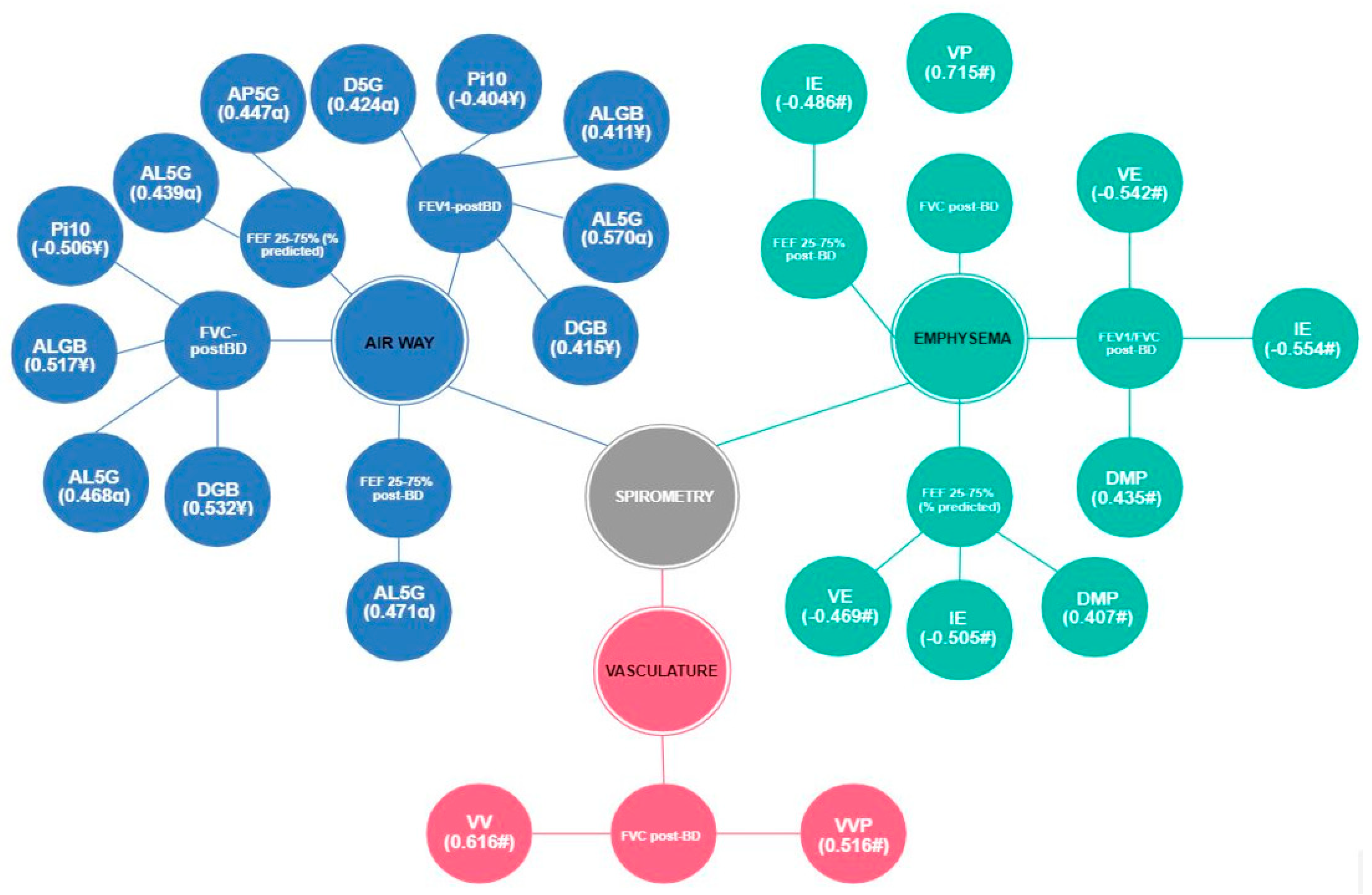
 —Moderate correlation;
—Moderate correlation;  —Strong correlation; Total MV—alveolar minute volume; VD/VT aw—ratio of airway dead space to expired volume; PeCO2—mean partial pressure of mean carbon dioxide; VT alv—alveolar tidal volume; ETCO2—partial pressure of carbon dioxide at end-tidal; Vi—volume inspired; Ve—volume expired; PSlp3—phase 3 slope; P3Slp/ETCO2—phase 3 slope normalized by ETCO2; P3Slp/Ve—phase 3 slope normalized by Ve; HR—heart rate; Ti—inspiratory time; Te—expiratory time; ALGB—lumen area by bronchial generation; AL3G—lumen area (3rd generation); AL5G—lumen area (5th generation); Pi10—internal perimeter of 10 mm; WGB—relative wall thickness by bronchial generation; W3G—relative wall thickness (3rd generation); W4G—relative wall thickness (4th generation); W5G—relative wall thickness (5th generation); AP5G—Mean peak attenuation (5th generation); EP5G—wall thickness (5th generation); DGB—diameter by bronchial generation; D5G—diameter (5th generation).
—Strong correlation; Total MV—alveolar minute volume; VD/VT aw—ratio of airway dead space to expired volume; PeCO2—mean partial pressure of mean carbon dioxide; VT alv—alveolar tidal volume; ETCO2—partial pressure of carbon dioxide at end-tidal; Vi—volume inspired; Ve—volume expired; PSlp3—phase 3 slope; P3Slp/ETCO2—phase 3 slope normalized by ETCO2; P3Slp/Ve—phase 3 slope normalized by Ve; HR—heart rate; Ti—inspiratory time; Te—expiratory time; ALGB—lumen area by bronchial generation; AL3G—lumen area (3rd generation); AL5G—lumen area (5th generation); Pi10—internal perimeter of 10 mm; WGB—relative wall thickness by bronchial generation; W3G—relative wall thickness (3rd generation); W4G—relative wall thickness (4th generation); W5G—relative wall thickness (5th generation); AP5G—Mean peak attenuation (5th generation); EP5G—wall thickness (5th generation); DGB—diameter by bronchial generation; D5G—diameter (5th generation).
 —Moderate correlation;
—Moderate correlation;  —Strong correlation; Total MV—alveolar minute volume; VD/VT aw—ratio of airway dead space to expired volume; PeCO2—mean partial pressure of mean carbon dioxide; VT alv—alveolar tidal volume; ETCO2—partial pressure of carbon dioxide at end-tidal; Vi—volume inspired; Ve—volume expired; PSlp3—phase 3 slope; P3Slp/ETCO2—phase 3 slope normalized by ETCO2; P3Slp/Ve—phase 3 slope normalized by Ve; HR—heart rate; Ti—inspiratory time; Te—expiratory time; ALGB—lumen area by bronchial generation; AL3G—lumen area (3rd generation); AL5G—lumen area (5th generation); Pi10—internal perimeter of 10 mm; WGB—relative wall thickness by bronchial generation; W3G—relative wall thickness (3rd generation); W4G—relative wall thickness (4th generation); W5G—relative wall thickness (5th generation); AP5G—Mean peak attenuation (5th generation); EP5G—wall thickness (5th generation); DGB—diameter by bronchial generation; D5G—diameter (5th generation).
—Strong correlation; Total MV—alveolar minute volume; VD/VT aw—ratio of airway dead space to expired volume; PeCO2—mean partial pressure of mean carbon dioxide; VT alv—alveolar tidal volume; ETCO2—partial pressure of carbon dioxide at end-tidal; Vi—volume inspired; Ve—volume expired; PSlp3—phase 3 slope; P3Slp/ETCO2—phase 3 slope normalized by ETCO2; P3Slp/Ve—phase 3 slope normalized by Ve; HR—heart rate; Ti—inspiratory time; Te—expiratory time; ALGB—lumen area by bronchial generation; AL3G—lumen area (3rd generation); AL5G—lumen area (5th generation); Pi10—internal perimeter of 10 mm; WGB—relative wall thickness by bronchial generation; W3G—relative wall thickness (3rd generation); W4G—relative wall thickness (4th generation); W5G—relative wall thickness (5th generation); AP5G—Mean peak attenuation (5th generation); EP5G—wall thickness (5th generation); DGB—diameter by bronchial generation; D5G—diameter (5th generation).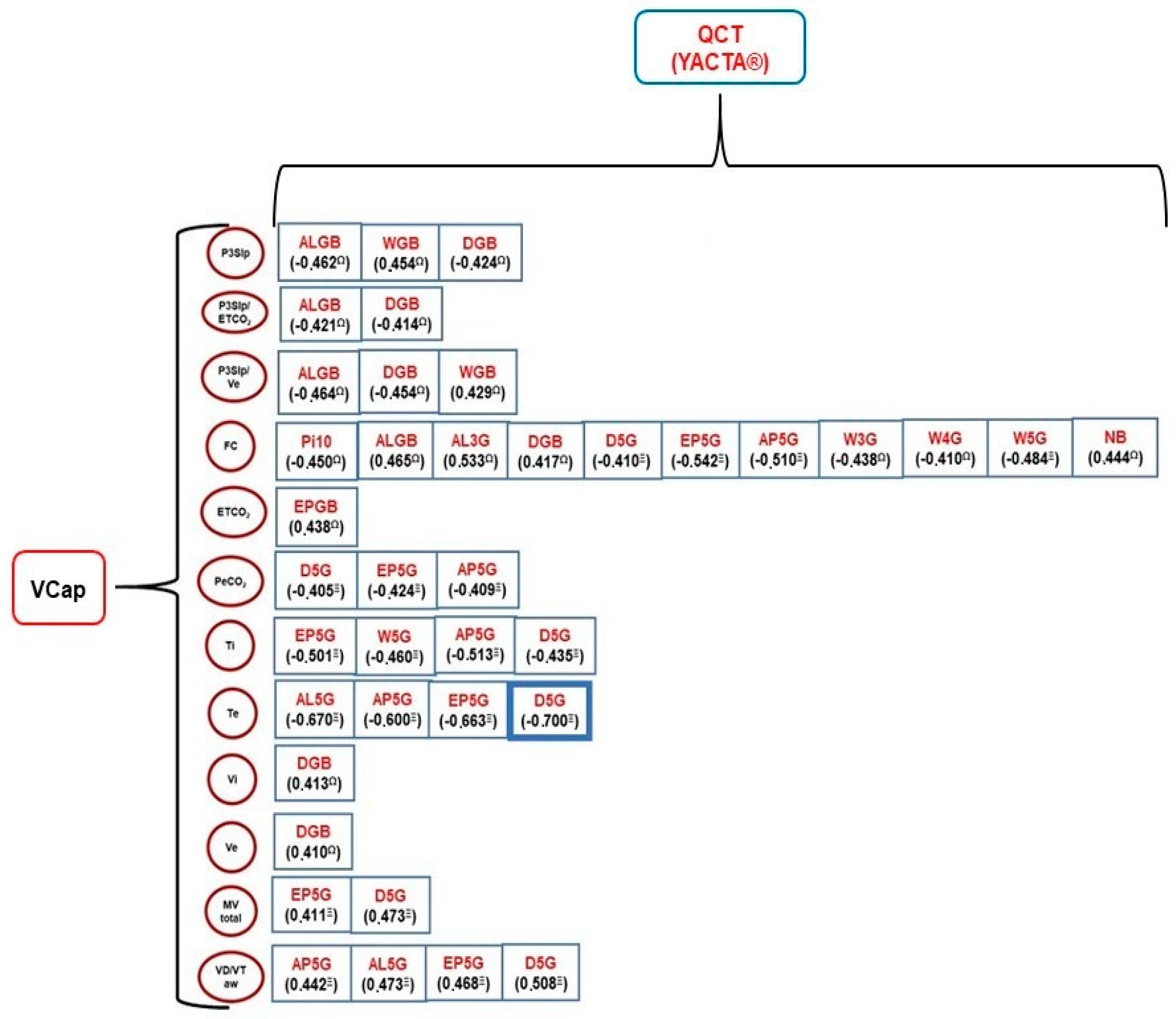
| N = 27 | Mean/SD | |
|---|---|---|
| Clinical data | Age (years) | 65.07 ± 8.25 |
| Cigarettes/day | 30.03 ± 17.05 | |
| Smoking time (years) | 40.37 ± 11.56 | |
| Years/pack | 58.57 ± 34.21 | |
| BMI (kg/m2) | 28.13 ± 5.94 | |
| Spirometry | FVC pre-BD (L) | 1.31 ± 0.61 |
| FVC % predicted pre-BD | 55.59 ± 11.73 | |
| FEV1 pre-BD (L) | 1.18 ± 0.5 | |
| FEV1 % predicted pre-BD | 42.22 ± 12.73 | |
| FEF 25–75% pre-BD | 0.69 ± 0.48 | |
| FVC post-BD (L) | 1.99 ± 0.6 | |
| FVC % post-BD | 57.54 ± 11.64 | |
| FEV1 post-BD (L) | 1.22 ± 0.45 | |
| FEV1 % predicted post-BD | 44.81 ± 12.34 | |
| FEV1/FVC post-BD | 16.36 ± 27.5 | |
| FEF 25–75% post-BD | 0.75 ± 0.49 | |
| 6MWT | Distance covered (m) | 345.3 ± 85.7 |
| Distance covered (% of predicted value) | 71 ± 0.14 | |
| Distance covered predicted | 471.31 ± 80.11 | |
| SpO2 baseline (%) | 94.96 ± 2.75 | |
| SpO2 6 min (%) | 87.77 ± 7.27 | |
| Desaturation (final SpO2—initial SpO2) | 7.03 ± 6.35 | |
| Volumetric capnography | Total MV (mL) | 7.9 ± 2.94 |
| Total alveolar MV (mL) | 5.7 ± 2.05 | |
| Total RR (cpm) | 17 ± 5.64 | |
| Vd aw (mL) | 131.5 ± 24.58 | |
| VD/VT aw | 0.29 ± 0.04 | |
| VCO2 (mL) | 172.1 ± 57.73 | |
| PeCO2 (mmHg) | 19.6 ± 4.13 | |
| ETCO2 (mmHg) | 34.2 ± 5.15 | |
| Vi (mL) | 466.9 ± 129.36 | |
| Ve (mL) | 482.2 ± 134.61 | |
| VCO2/br (mL/breathing) | 10.7 ± 4.9 | |
| P2Slp (mmHg) | 280.32 ± 89.30 | |
| P3Slp (mmHg) | 38.67 ± 19.66 | |
| P3Slp/ETCO2 | 1.11 ± 0.51 | |
| P3Slp/Ve | 0.11 ± 0.08 | |
| VT alv (mL) | 358.9 ± 138.8 | |
| HR baseline (bpm) | 83.22 ± 11.04 | |
| Ti (sec) | 1.47 ± 0.62 | |
| Te (sec) | 2.46 ± 1.35 |
| Variables | COPD (N Variable, See Legend) | Control (N = 19) | p | |
|---|---|---|---|---|
| Computed Tomography | ||||
| Airways | ERPB3_8 (%) | 54.85 ± 7.71 ¥ | 46.19 ± 7.69 | 0.0012 1 |
| ERPB (%) | 53.06 ± 4.88 ¥ | 46.37 ± 4.68 | 0.0001 1 | |
| Pi10 | 0.30 ± 0.14 ¥ | 0.19 ± 0.11 | 0.0079 1 | |
| W3G (%) | 57.85 ± 7.88 ¥ | 51.84 ± 5.21 | 0.0013 1 | |
| W4G (%) | 60.43 ± 6.89 ¥ | 53.91 ± 6.12 | 0.0029 1 | |
| W5G (%) | 60.94 ± 7.24 α | 51.03 ± 7.80 | 0.0002 1 | |
| AP4G (UH) | −255.67 ± 100.73 ¥ | −335.86 ± 77.46 | 0.0069 1 | |
| AP5G (UH) | −317.24 ± 132.05 α | −450.93 ± 91.69 | 0.0006 1 | |
| EP3G (mm2) | 2.22 ± 0.45 ¥ | 2.00 ± 0.24 | 0.0428 1 | |
| EP4G (mm2) | 1.82 ± 0.34 ¥ | 1.58 ± 0.29 | 0.0263 1 | |
| EP5G (mm2) | 1.66 ± 0.53 α | 1.21 ± 0.31 | 0.0014 1 | |
| Emphysema | VE (cm3) | 1195.59 ± 1067.32 # | 11.00 ± 17.65 | <0.0001 1 |
| IE (%) | 21.93 ± 17.99 # | 0.11 ± 0.32 | <0.0001 1 | |
| DMP (UH) | −841.33 ± 40.43 # | −797.16 ± 27.87 | 0.0002 1 | |
| Spirometry | ||||
| FVC pre-BD (L) | 1.91 ± 0.63 # | 4.45 ± 0.87 | <0.0001 1 | |
| FEV1 pre-BD (L) | 1.19 ± 0.51 # | 3.70 ± 0.73 | <0.0001 1 | |
| FEV1/FVC pre-BD | 0.60 ± 0.12 # | 0.91 ± 0.20 | <0.0001 1 | |
| FEF 25–75% pre-BD | 0.69 ± 0.49 # | 4.31 ± 1.49 | <0.0001 1 | |
| Clinical Variables | CT Scan Data | Correlation (N Variable, See Legend) | p | |
|---|---|---|---|---|
| Airways | ||||
| Cigarette/day | versus | WGB (%) | −0.41196 ¥ | 0.0365 |
| W3G (%) | −0.43891 ¥ | 0.0249 | ||
| EP5G (mm2) | −0.41731 α | 0.0598 | ||
| Smoking time (years) | versus | AL3G (mm2) | −0.47476 ¥ | 0.0143 |
| Emphysema | ||||
| BMI (kg/m2) | versus | VE (cm3) | −0.51960 # | 0.0055 |
| IE (%) | −0.54213 # | 0.0035 | ||
| Vasculature | ||||
| Age (years) | versus | VV (cm3) | −0.52400 ¤ | 0.0086 |
| 6MWT | CT Scan Data | Correlation (N Variable, See Legend) | p | |
|---|---|---|---|---|
| Airways | ||||
| Distance covered (meters) | versus | W5G (%) | −0.50829 α | 0.0186 |
| Vasculature | ||||
| Distance covered (% of predicted distance) | versus | VV (cm3) | −0.49130 ¤ | 0.0148 |
| VVP (cm3) | −0.44967 ¤ | 0.0275 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diniz, O.H.G.; Pereira, M.C.; da Silva, S.M.D.; Koenigkam-Santos, M.; Paschoal, I.A.; Moreira, M.M. Correlations between Volumetric Capnography and Automated Quantitative Computed Tomography Analysis in Patients with Severe COPD. J. Respir. 2022, 2, 13-24. https://doi.org/10.3390/jor2010002
Diniz OHG, Pereira MC, da Silva SMD, Koenigkam-Santos M, Paschoal IA, Moreira MM. Correlations between Volumetric Capnography and Automated Quantitative Computed Tomography Analysis in Patients with Severe COPD. Journal of Respiration. 2022; 2(1):13-24. https://doi.org/10.3390/jor2010002
Chicago/Turabian StyleDiniz, Odair Henrique Gaverio, Monica Corso Pereira, Silvia Maria Doria da Silva, Marcel Koenigkam-Santos, Ilma Aparecida Paschoal, and Marcos Mello Moreira. 2022. "Correlations between Volumetric Capnography and Automated Quantitative Computed Tomography Analysis in Patients with Severe COPD" Journal of Respiration 2, no. 1: 13-24. https://doi.org/10.3390/jor2010002







