A Qualitative Study Exploring the Impact and Effects Following Hospital Discharge of COVID-19
Abstract
:1. Introduction
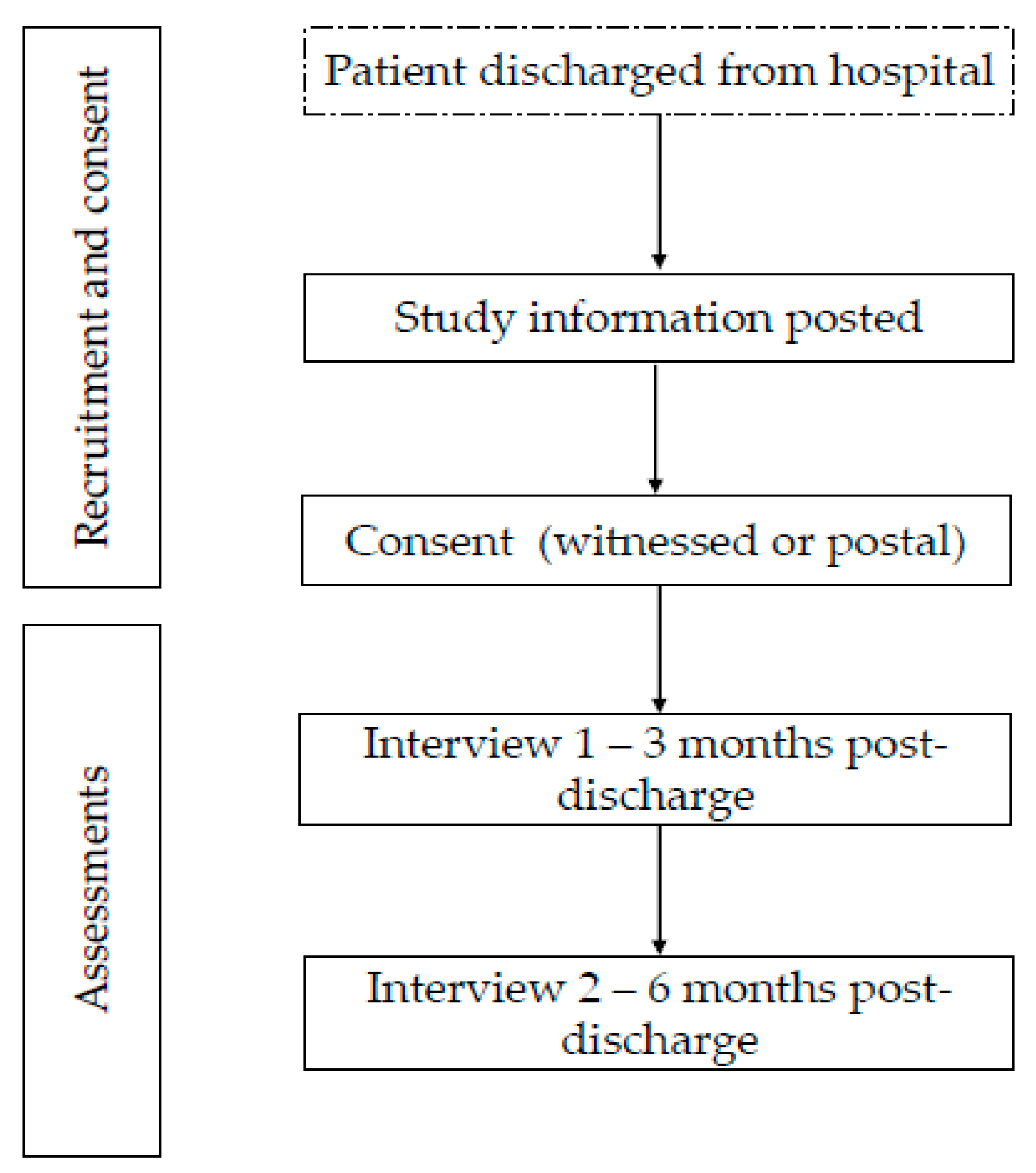
2. Materials and Methods
2.1. Study Design
Aims and Objectives
- To understand the experiences of patients who have been hospitalized with COVID-19;
- To understand the impact of COVID-19 on patients’ QoL;
- To understand longer-term impacts of COVID-19 on patients QoL to guide the development of appropriate interventions and services to meet the needs of patients.
2.2. Participants
- Patients aged ≥18 years hospitalized with COVID-19 (polymerase chain reaction [PCR] swab positivity for SARS-CoV-2 and a clinical diagnosis of COVID-19 according to the admitting medical team;
- Discharged from hospital;
- Ability to provide informed consent;
- Willingness to take part in the interviews over the phone or by video call;
- Inability to give informed consent.
2.2.1. Sampling Technique and Identification
2.2.2. Consent
2.3. Study Interventions
2.4. Risk of Bias
2.5. Patient and Public Involvement
2.6. Data Analysis
2.7. Approvals
2.7.1. Data Handling and Storage
2.7.2. Adverse Events
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Interview Questions |
|---|
|
References
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 31 July 2021).
- Zhou, Z.; Zhang, M.; Wang, Y.; Zheng, F.; Huang, Y.; Huang, K.; Yu, Q.; Cai, C.; Chen, D.; Tian, Y.; et al. Clinical characteristics of older and younger patients infected with SARS-CoV-2. Aging (Albany NY) 2020, 12, 11296–11305. [Google Scholar] [CrossRef] [PubMed]
- Shahid, Z.; Kalayanamitra, R.; McClafferty, B.; Kepko, D.; Ramgobin, D.; Patel, R.; Aggarwal, C.S.; Vunnam, R.; Sahu, N.; Bhatt, D.; et al. COVID-19 and older adults: What we know. J. Am. Geriatr. Soc. 2020, 68, 926–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- COVID-19 Map—Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 31 July 2021).
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef] [PubMed]
- Rees, E.M.; Nightingale, E.S.; Jafari, Y.; Waterlow, N.R.; Clifford, S.; Pearson, C.A.B.; CMMID Working Group; Jombart, T.; Procter, S.R.; Knight, G.M. COVID-19 length of hospital stay: A systematic review and data synthesis. BMC Med. 2020, 18, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Garner, P. For 7 Weeks I Have Been Through a Roller Coaster of Ill Health, Extreme Emotions, and Utter Exhaustion; BMJ Opinion: London, UK, 2020. [Google Scholar]
- Mahase, E. Covid-19: What do we know about “long covid”? BMJ 2020, 370, m2815. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F. For the Gemelli Against COVID-19 Post-Acute Care Study Group Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J. Med. Virol. 2020, 93, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Liu, T.B.; Chen, X.Y.; Miao, G.D.; Zhang, L.; Zhang, Q.; Cheung, T. Recommendations on diagnostic criteria and prevention of SARS-related mental disorders. J. Clin. Psychol. Med. 2003, 13, 188–191. [Google Scholar]
- Maunder, R.; Hunter, J.; Vincent, L.; Bennett, J.; Peladeau, N.; Leszcz, M.; Sadavoy, J.; Verhaeghe, L.M.; Steinberg, R.; Mazzulli, T. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. Can. Med. Assoc. J. 2003, 168, 1245–1251. [Google Scholar]
- Hu, Y.; Chen, Y.; Zheng, Y.; You, C.; Tan, J.; Hu, L.; Zhang, Z.; Ding, L. Factors related to mental health of inpatients with COVID-19 in Wuhan, China. Brain Behav. Immun. 2020, 89, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.T.; Yang, Y.; Li, W.; Zhang, L.; Zhang, Q.; Cheung, T.; Ng, C.H. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry 2020, 7, 228–229. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.K.; Webster, R.; E Smith, L.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Windle, K.; Francis, J.; Coomber, C. Preventing Loneliness and Social Isolation: Interventions and Outcomes; Social Care Institute for Excellence: London, UK, 2011. [Google Scholar]
- Boyes, R. Patient perspective: Roger Boyes. BMJ 2020, 369, m1813. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, K.; Barnett, J.; Thorpe, S.; Young, T. Characterising and justifying sample size sufficiency in interview-based studies: Systematic analysis of qualitative health research over a 15-year period. BMC Med. Res. Methodol. 2018, 18, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Braun, V.; Clarke, V. What can “thematic analysis” offer health and wellbeing researchers? Int. J. Qual. Stud. Health Well-Being 2014, 9, 26152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reay, A.; Aujayeb, A.; Dotchin, C.; Tullo, E.; Steer, J.; Swainston, K.; Dismore, L. A Qualitative Study Exploring the Impact and Effects Following Hospital Discharge of COVID-19. J. Respir. 2021, 1, 216-222. https://doi.org/10.3390/jor1030020
Reay A, Aujayeb A, Dotchin C, Tullo E, Steer J, Swainston K, Dismore L. A Qualitative Study Exploring the Impact and Effects Following Hospital Discharge of COVID-19. Journal of Respiration. 2021; 1(3):216-222. https://doi.org/10.3390/jor1030020
Chicago/Turabian StyleReay, Abigail, Avinash Aujayeb, Catherine Dotchin, Ellen Tullo, John Steer, Katherine Swainston, and Lorelle Dismore. 2021. "A Qualitative Study Exploring the Impact and Effects Following Hospital Discharge of COVID-19" Journal of Respiration 1, no. 3: 216-222. https://doi.org/10.3390/jor1030020
APA StyleReay, A., Aujayeb, A., Dotchin, C., Tullo, E., Steer, J., Swainston, K., & Dismore, L. (2021). A Qualitative Study Exploring the Impact and Effects Following Hospital Discharge of COVID-19. Journal of Respiration, 1(3), 216-222. https://doi.org/10.3390/jor1030020






