Characterization of a Cohort of Patients with Chronic Thromboembolic Pulmonary Hypertension from Northeastern Colombia (REHINO Study)
Abstract
1. Introduction
2. Methods
2.1. Ethical Considerations
2.2. Study Design
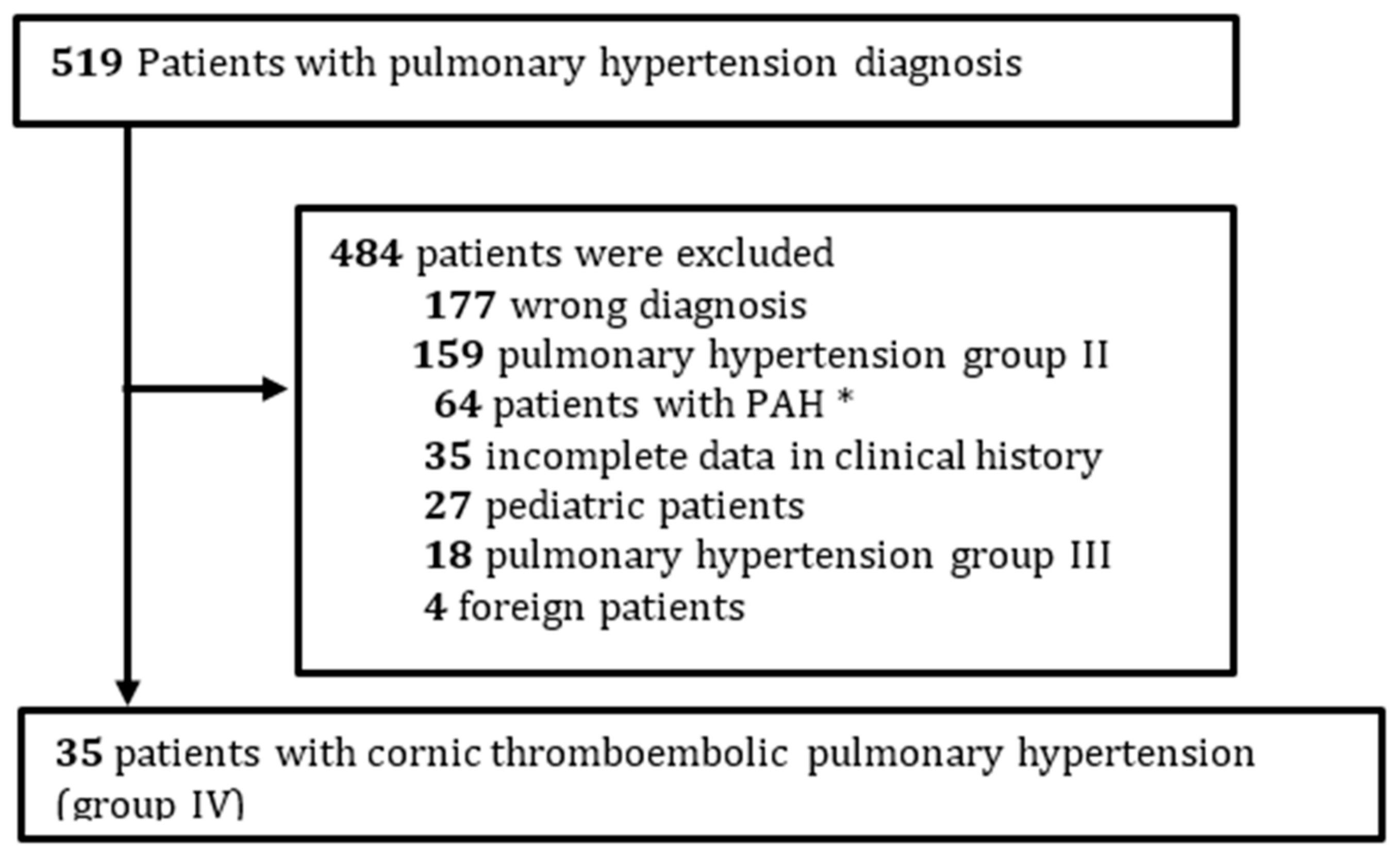
2.3. Patients
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Elwing, J.M.; Vaidya, A.; Auger, W.R. Chronic Thromboembolic Pulmonary Hypertension. Clin. Chest Med. 2018, 39, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Delcroix, M.; Jais, X.; Madani, M.M.; Matsubara, H.; Mayer, E.; Ogo, T.; Tapson, V.F.; Ghofrani, H.-A.; Jenkins, D.P. Chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801915. [Google Scholar] [CrossRef] [PubMed]
- Pengo, V.; Lensing, A.W.; Prins, M.H.; Marchiori, A.; Davidson, B.L.; Tiozzo, F.; Albanese, P.; Biasiolo, A.; Pegoraro, C.; Iliceto, S.; et al. Incidence of Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Embolism. N. Engl. J. Med. 2004, 350, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Couturaud, F.; Delcroix, M.; Humbert, M. Diagnosis of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Eur. Respir. J. 2020, 55, 2000189. [Google Scholar] [CrossRef] [PubMed]
- Verano, R.J.; Rojas, M.X.; Molina, Á.; Roa, J.; Granados, M.; Londoño, A.; Tobón, L.I.; Dueñas, C.; Rodríguez, M.N.; González, M.; et al. Curso clínico y supervivencia en embolia pulmonar. Acta Médica Colomb. 2008, 33, 111–116. [Google Scholar]
- Pablo, M.; Guzman, R.; Baños, I.; Alvarez, A. Epidemiologia de la hipertension pulmonar en colombia Epidemiology of pulmonary hypertension in colombia. Salud Uninorte 2018, 34, 607–624. [Google Scholar]
- Auger, W.R. Surgical and Percutaneous Interventions for Chronic Thromboembolic Pulmonary Hypertension. Cardiol. Clin. 2020, 38, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.-A.; D’Armini, A.M.; Grimminger, F.; Hoeper, M.M.; Jansa, P.; Kim, N.H.; Mayer, E.; Simonneau, G.; Wilkins, M.R.; Fritsch, A.; et al. Riociguat for the Treatment of Chronic Thromboembolic Pulmonary Hypertension. N. Engl. J. Med. 2013, 369, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.-A.; Simonneau, G.; D’Armini, A.M.; Fedullo, P.; Howard, L.S.; Jaïs, X.; Jenkins, D.P.; Jing, Z.-C.; Madani, M.M.; Martin, N.; et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): Results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir. Med. 2017, 5, 785–794. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiéry, J.-L.; Gibbs, S.; Lang, I.M.; Kaminski, K.A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2015, 37, 67–119. [Google Scholar] [CrossRef] [PubMed]
- Jaïs, X.; D’Armini, A.M.; Jansa, P.; Torbicki, A.; Delcroix, M.; Ghofrani, H.A.; Hoeper, M.M.; Lang, I.M.; Mayer, E.; Pepke-Zaba, J.; et al. Bosentan for Treatment of Inoperable Chronic Thromboembolic Pulmonary Hypertension. J. Am. Coll. Cardiol. 2008, 52, 2127–2134. [Google Scholar] [CrossRef] [PubMed]
- Villaquirán-Torres, C. Evaluación diagnóstica en hipertensión arterial pulmonar. Rev. Colomb. Cardiol. 2017, 24, 20–27. [Google Scholar] [CrossRef]
- Escribano-Subias, P.; Blanco, I.; López-Meseguer, M.; Lopez-Guarch, C.J.; Roman, A.; Morales, P.; Castillo-Palma, M.J.; Segovia, J.; Gómez-Sanchez, M.A.; Barberà, J.A. Survival in pulmonary hypertension in Spain: Insights from the Spanish registry. Eur. Respir. J. 2012, 40, 596–603. [Google Scholar] [CrossRef]
- Villaquirán-torres, C. Hipertensión arterial pulmonar en Bogotá: Descripción de un grupo de pacientes pertenecientes al Programa Institucional de la Fundación Neumológica Colombiana. Rev. Colomb. Neumol. 2010, 22, 3–10. [Google Scholar]
- Mahmud, E.; Madani, M.M.; Kim, N.H.; Poch, D.; Ang, L.; Behnamfar, O.; Patel, M.P.; Auger, W.R. Chronic Thromboembolic Pulmonary Hypertension. J. Am. Coll. Cardiol. 2018, 71, 2468–2486. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Madani, M.; Fadel, E.; D’Armini, A.M.; Mayer, E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160111. [Google Scholar] [CrossRef] [PubMed]
- Van Thor, M.C.J.; Klooster, L.T.; Snijder, R.J.; Kelder, J.C.; Mager, J.J.; Post, M.C. Bosentan or Macitentan Therapy in Chronic Thromboembolic Pulmonary Hypertension? Lung 2019, 197, 753–760. [Google Scholar] [CrossRef]
- Consulta de datos de producto. Instituto Nacional de Vigilancia de Medicamentos y Alimentos. 2020. Available online: http://consultaregistro.invima.gov.co:8082/Consultas/consultas/consreg_encabcum.jsp (accessed on 1 November 2020).
- Wilkens, H.; Konstantinides, S.; Lang, I.M.; Bunck, A.C.; Gerges, M.; Gerhardt, F.; Grgic, A.; Grohé, C.; Guth, S.; Held, M.; et al. Chronic thromboembolic pulmonary hypertension (CTEPH): Updated Recommendations from the Cologne Consensus Conference 2018. Int. J. Cardiol. 2018, 272, 69–78. [Google Scholar] [CrossRef]
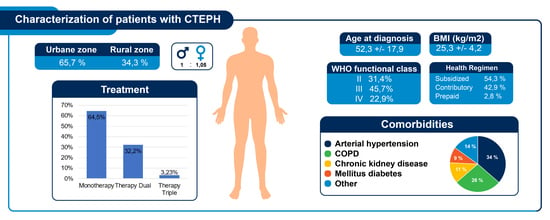
| Variable | N | Mean ± SD or (%) |
|---|---|---|
| Age at diagnosis [years] | 35 | 52.3 ± 17.9 |
| Time from onset of symptoms to diagnosis (months) | 35 | 13.8 ± 15 |
| Gender (Male n (%)) | 35 | 17 (48.6%) |
| BMI (kg/m2) | 29 | 25.3 ± 4.2 |
| Alive at enrolment time | 35 | 27 (77.1%) |
| Location | ||
| Urban area | 35 | 23 (65.7%) |
| Rural area | 35 | 12 (34.3%) |
| Past medical history | ||
| Pulmonary embolism | 25 (71.4%) | |
| Deep venous thrombosis | 13 (37.1%) | |
| Thrombophilia | 12 (34.3%) | |
| Antiphospholipid syndrome | 8 (66%) | |
| Protein C deficiency | 1 (8.3%) | |
| Protein S deficiency | 1 (8.3%) | |
| Antithrombin III deficiency | 1 (8.3%) | |
| No past medical history | 1 (8.3%) | |
| Cancer | 0 | |
| Comorbidities | ||
| Arterial hypertension | 12 (34.3%) | |
| COPD | 9 (25.7%) | |
| Chronic kidney disease | 4 (11.8%) | |
| Diabetes Mellitus | 3 (8.6%) | |
| Coronary heart disease | 1 (2.8%) | |
| Interstitial lung disease | 1 (2.8%) | |
| Dyslipidemia | 1 (2.8%) | |
| Cerebrovascular disease | 1 (2.8%) | |
| Atrial fibrillation | 1 (2.8%) | |
| Functional class according to WHO | ||
| II | 11 (31.4%) | |
| III | 16 (45.7%) | |
| IV | 8 (22.9%) | |
| Symptoms | ||
| Dyspnea | 35 (100%) | |
| Chest pain | 20 (57%) | |
| Syncope | 8 (22.8%) | |
| Clinical parameters | ||
| BNP [pg/mL] | 14 | 338 ± 342 |
| Computed tomography Angiogram (CT-Angiogram) | 35 | 30 (85.7%) |
| Pulmonary ventilation/perfusion scan | 35 | 15 (42.8%) |
| 6 min walking test (6MWT) (meters) | 7 | 365.3 ± 127 |
| VO2 Max (ml/kg/min) | 6 | 12.9 ± 3.8 |
| VE/VCO2 | 6 | 43.4 ± 9.1 |
| Variable | N | Mean ± SD or (%) |
|---|---|---|
| Basal echocardiography | ||
| LVEF (%) | 35 | 58.2 ± 5.2 |
| TAPSE (centimeters) | 16 | 13.5 ± 3.8 |
| Pulmonary systolic pressure (mmHg) | 35 | 86.2 ± 20.9 |
| Pericardial effusion | 35 | 7 (20.6%) |
| Hemodynamics measured by right cardiac catheterization. | ||
| Mean PAP (mmHg) | 35 | 48.9 ± 10.7 |
| MPAP 25–30 (mmHg) | 2 (5.7%) | |
| MPAP 30–35 (mmHg) | 2 (5.7%) | |
| MPAP > 35 (mmHg) | 31 (88.5%) | |
| Wedge pressure of the pulmonary artery (mmHg) | 26 | 15.3 ± 7.2 |
| Cardiac output (L/min) | 25 | 3.9 ± 1.4 |
| Cardiac index (L/min/m2 median –RIQ) | 25 | 1.89 (RIQ 1.38) |
| Pulmonary vascular resistance (U Wood) | 30 | 13.5 ± 8.4 |
| Pulmonary vascular resistance (U Wood) > 12 | 15 (50%) |
| Vasodilator Drug | N | n (%) |
|---|---|---|
| Patients under pharmacological therapy | 35 | 31 (89%) |
| Monotherapy | 20 | 20 (64.5%) |
| Bosentan | 12 (60%) | |
| Sildenafil | 5 (25%) | |
| Riociguat | 2 (10%) | |
| Nifedipine | 1 (5%) | |
| Therapy Dual | 10 | 10 (32.2%) |
| Sildenafil + Bosentan | 5 (50%) | |
| Riociguat + Bosentan | 1 (10%) | |
| Sildenafil + Nifedipine | 1 (10%) | |
| Sildenafil + Iloprost | 1 (10%) | |
| Bosentan + Iloprost | 1 (10%) | |
| Nifedipine + Bosentan | 1 (10%) | |
| Therapy Triple | 1 | 1 (100%) |
| Sildenafil + Nifedipine + Bosentan | 1 (100%) |
| Variable | CTEPH | ||
|---|---|---|---|
| N | Basal Average ± SD o n y (%) | Follow-up Average ± SD on y (%) | |
| LVEF (%) | 11 | 61.5 ± 5.6 | 58.4 ± 5.7 |
| Pulmonary systolic pressure (mmHg) | 9 | 78.9 ± 18.0 | 59.0 ± 24.4 ** |
| Hemodynamics measured by right cardiac catheterization | |||
| Pulmonary artery systolic pressure (mmHg) | 11 | 89.5 ± 16.1 | 69.5 ± 28.0 |
| Diastolic pressure of the pulmonary artery (mmHg) | 11 | 29.9 ± 8.2 | 23.3 ± 11.0 |
| Mean pulmonary arterial pressure (mmHg) | 10 | 51.6 ± 7.2 | 43.3 ± 16.48 |
| Wedge pressure of the pulmonary artery (mmHg) | 7 | 15.3 ± 5.3 | 14.3 ± 8.2 |
| Cardiac output (L / min) | 8 | 4.1 ± 1.9 | 4.6 ± 1.7 |
| Pulmonary vascular resistance (U Wood) | 9 | 14.3 ± 9.2 | 7.0 ± 5.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fajardo-Rivero, J.E.; Mogollón, M.; García-Bohórquez, D.F.; Villabona-Rueda, A.; Mendoza-Herrera, T.; Ramírez-Sarmiento, A.; Bolívar-Grimaldos, F.; Orozco-Levi, M. Characterization of a Cohort of Patients with Chronic Thromboembolic Pulmonary Hypertension from Northeastern Colombia (REHINO Study). J. Respir. 2021, 1, 105-113. https://doi.org/10.3390/jor1020012
Fajardo-Rivero JE, Mogollón M, García-Bohórquez DF, Villabona-Rueda A, Mendoza-Herrera T, Ramírez-Sarmiento A, Bolívar-Grimaldos F, Orozco-Levi M. Characterization of a Cohort of Patients with Chronic Thromboembolic Pulmonary Hypertension from Northeastern Colombia (REHINO Study). Journal of Respiration. 2021; 1(2):105-113. https://doi.org/10.3390/jor1020012
Chicago/Turabian StyleFajardo-Rivero, Javier Enrique, Melissa Mogollón, Diego Fernando García-Bohórquez, Andrés Villabona-Rueda, Tania Mendoza-Herrera, Alba Ramírez-Sarmiento, Fabio Bolívar-Grimaldos, and Mauricio Orozco-Levi. 2021. "Characterization of a Cohort of Patients with Chronic Thromboembolic Pulmonary Hypertension from Northeastern Colombia (REHINO Study)" Journal of Respiration 1, no. 2: 105-113. https://doi.org/10.3390/jor1020012
APA StyleFajardo-Rivero, J. E., Mogollón, M., García-Bohórquez, D. F., Villabona-Rueda, A., Mendoza-Herrera, T., Ramírez-Sarmiento, A., Bolívar-Grimaldos, F., & Orozco-Levi, M. (2021). Characterization of a Cohort of Patients with Chronic Thromboembolic Pulmonary Hypertension from Northeastern Colombia (REHINO Study). Journal of Respiration, 1(2), 105-113. https://doi.org/10.3390/jor1020012