The Current Landscape of Molecular Pathology for the Diagnosis and Treatment of Ependymoma
Abstract
1. Introduction
2. Histopathology and Molecular Pathology in Diagnosis of EPN
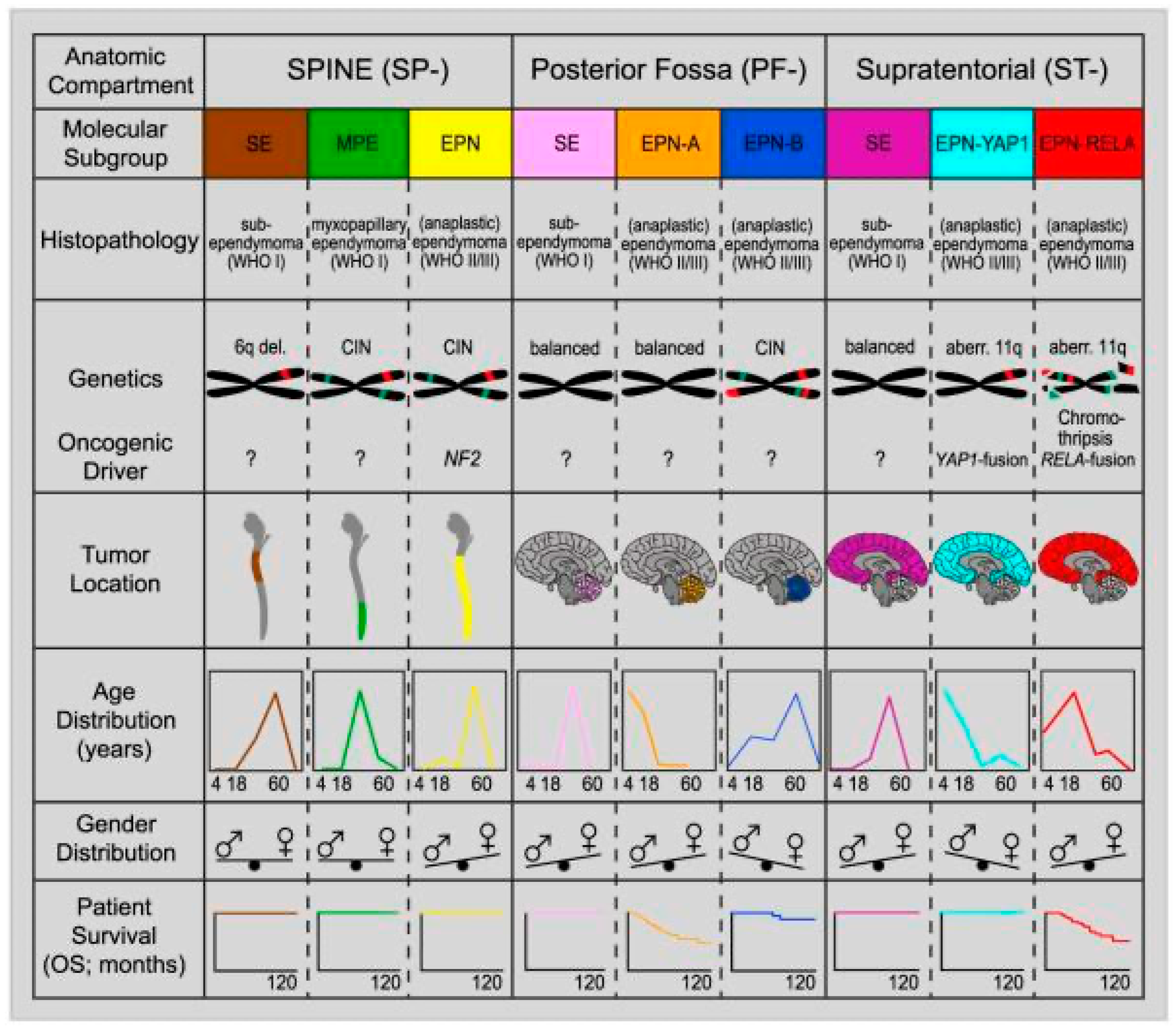
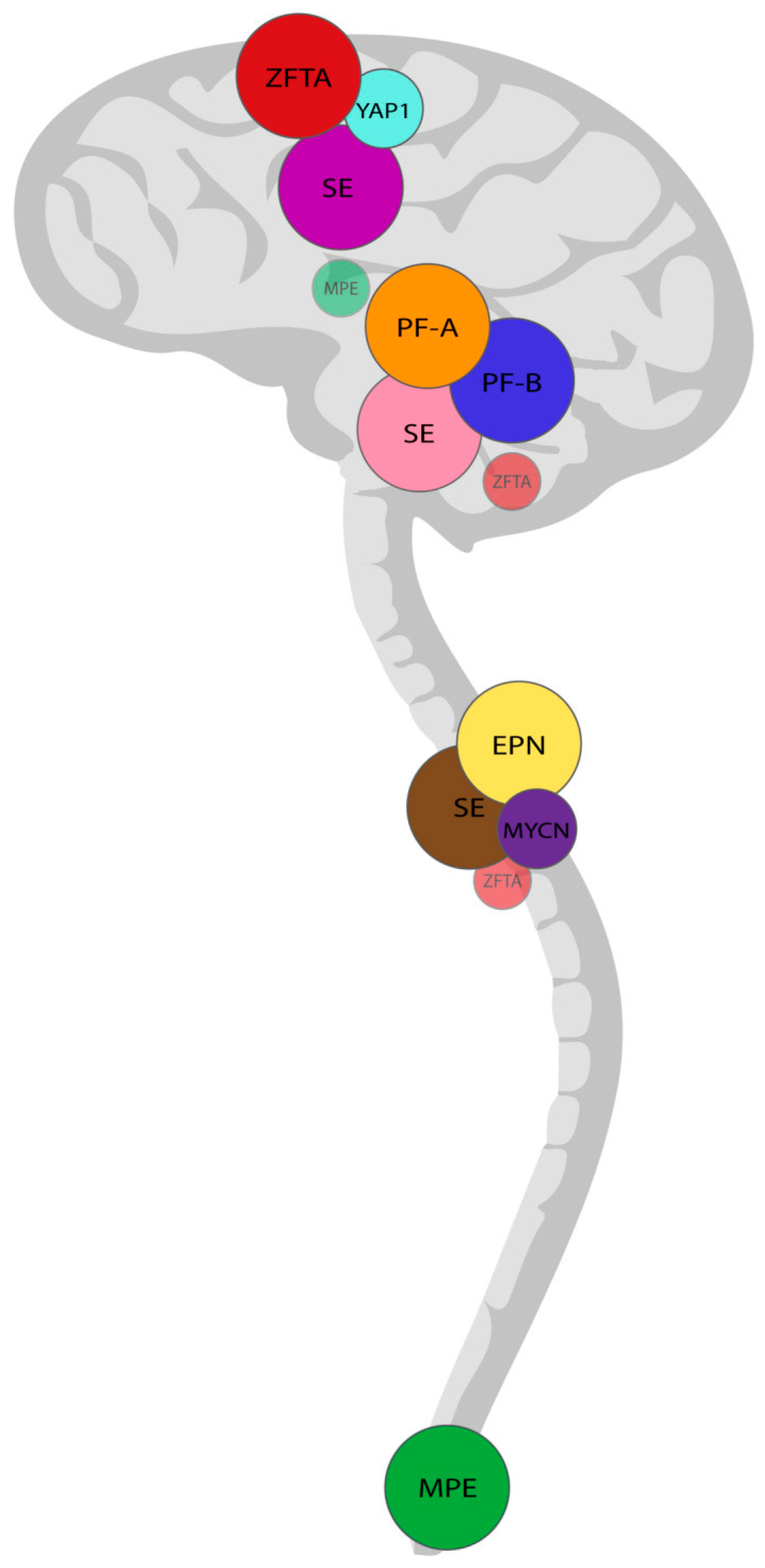
3. Grading of Ependymal Tumors
4. Supratentorial Ependymomas
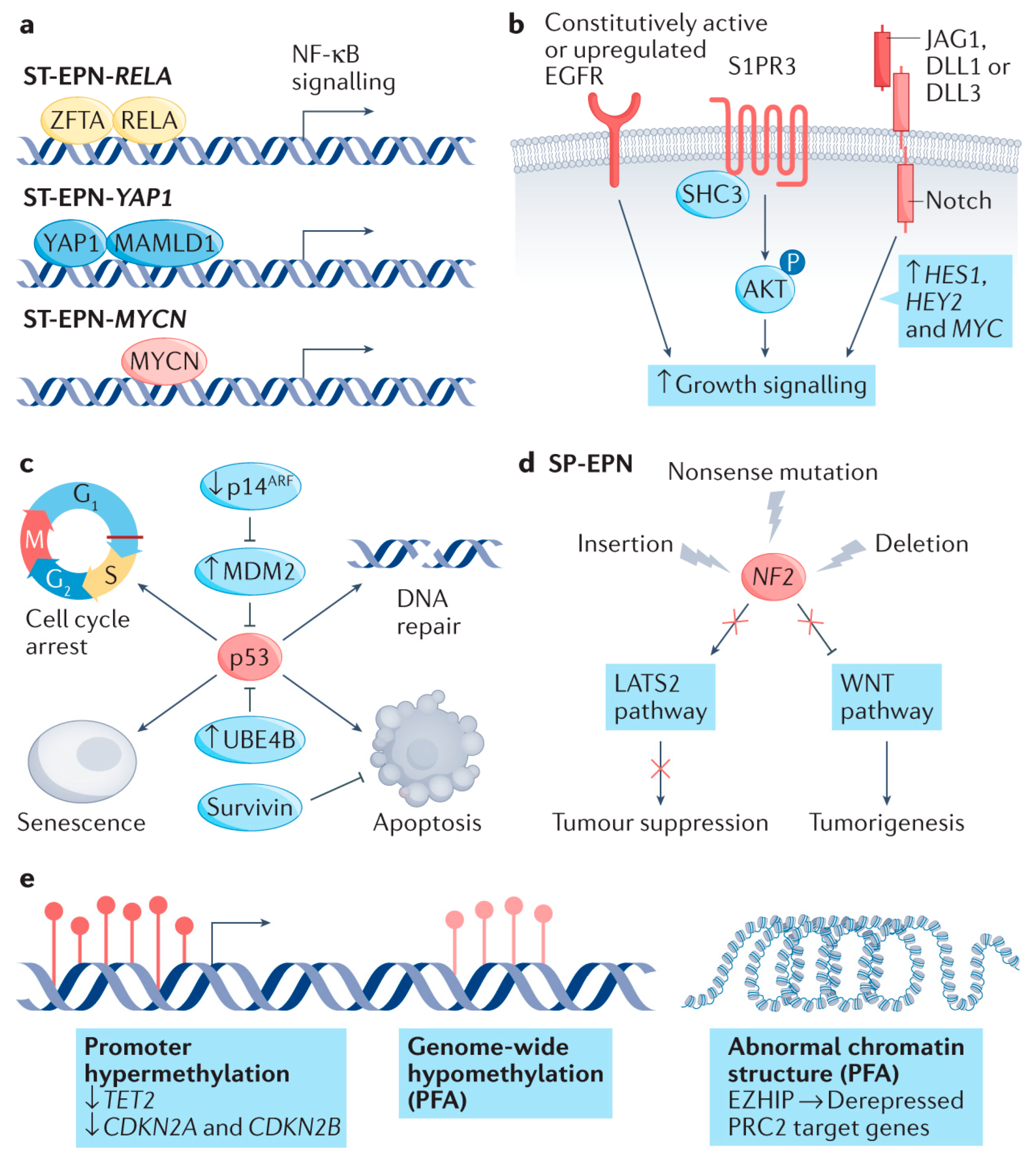
5. ZFTA-Fusion Positive Ependymomas
6. YAP1-Fusion Ependymomas
7. Posterior Fossa Ependymomas
8. PF-A Ependymomas
9. PF-B Ependymomas
10. Spinal Ependymomas
11. Spinal Ependymomas with MYCN Amplification
12. Myxopapillary Ependymomas
13. Subependymomas
14. Therapeutic Implications and Limitations to Current Standard of Care
15. Historical Clinical Trials
16. Ongoing Research to Refine Treatment
| Trial/Study Name | Status | Years | Focus/Design | Molecular Subtypes/Findings | Impact on Classification/Management | References |
|---|---|---|---|---|---|---|
| Genome-wide Methylation Profiling | Completed | 2011–2014 | Retrospective molecular profiling | Identified PFA, PFB, ST-ZFTA, ST-YAP1 | Established molecular subgroups, prognostic value | [66,78,79] |
| ACNS0831 (COG) | Completed | 2009–2019 | Randomized, maintenance chemo vs. observation | Not molecularly stratified (retrospective analyses performed) | No benefit of maintenance chemo; informed SIOP EPII design | NCT01096368 [74] |
| Large-Scale Methylation Studies | Completed | 2018–2023 | Multicenter, outcome correlation | All subtypes | Validated molecular subgroups, improved risk stratification | [66,78,79] |
| cIMPACT-NOW Update 7 | Completed | 2020 | Consensus classification update | ZFTA, YAP1, PFA, PFB, MYCN | Formalized molecular classification, dropped anaplastic grading | [11] |
| WHO CNS5 (2021) | Completed | 2021 | Classification update | Ten molecularly defined subtypes | Standardized molecular nomenclature, clinical relevance | [4] |
| SIOP Ependymoma II | Ongoing | 2014– | Prospective, molecularly stratified, international | Centralized molecular stratification (PFA, PFB, ST-ZFTA, ST-YAP1) | Integrates molecular data into therapy, biomarker-driven management | NCT02265770 [77] |
| BIOMECA (SIOP EPII) | Ongoing | 2020– | Prospective biomarker validation | PFA, PFB, ST-ZFTA, ST-YAP1 | Validated H3K27me3 IHC for PFA, fusion detection methods | NCT02265770 [80] |
17. Discussion
18. Future Directions
- Routine longitudinal molecular profiling at diagnosis and recurrence to capture tumor evolution and guide treatment adaptation.
- Functional validation of key oncogenic drivers to elucidate mechanisms of tumor initiation, progression, and resistance.
- Development of robust, subtype-specific preclinical models to support rational drug development and testing.
- Prospective, molecularly stratified clinical trials with clearly defined biologically relevant endpoints to identify targeted therapeutic opportunities.
- Standardization and global accessibility of molecular diagnostics through investments in infrastructure, training, and cost-containment strategies.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CNS | Central Nervous System |
| GFAP | glial fibrillary acidic protein |
| SE | subependymoma |
| PF-A | Posterior fossa group A |
| PF-B | Posterior fossa group B |
| MYCN | MYCN-amplified |
| MPE | Myxopapillary ependymomas |
| ZFTA | Zinc Finger Translocation Associated |
| YAP1 Yes | associated protein 1 |
| H3K27me3 | trimethylated histone H3 at lysine 27 |
| SP-SE | Spinal Subependymoma |
| PF | Posterior Fossa Subependymoma |
| ST–SE | Supratentorial Subependymoma |
| CERN | Collaborative Ependymoma Research Network |
| HDAC | histone deacetylases |
References
- Damodharan, S.; Puccetti, D. Pediatric Central Nervous System Tumor Overview and Emerging Treatment Considerations. Brain Sci. 2023, 13, 1106. [Google Scholar] [CrossRef]
- Jünger, S.T.; Timmermann, B.; Pietsch, T. Pediatric ependymoma: An overview of a complex disease. Child’s Nerv. Syst. 2021, 37, 2451–2463. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Mack, S.C.; Ramaswamy, V.; Smith, C.A.; Witt, H.; Smith, A.; Hansford, J.R.; von Hoff, K.; Wright, K.D.; Hwang, E.; et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2017, 133, 5–12. [Google Scholar] [CrossRef]
- Kresbach, C.; Neyazi, S.; Schüller, U. Updates in the classification of ependymal neoplasms: The 2021 WHO Classification and beyond. Brain Pathol. 2022, 32, e13068. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Witt, H.; Sill, M.; Jones, D.T.; Hovestadt, V.; Kratochwil, F.; Wani, K.; Tatevossian, R.; Punchihewa, C.; Johann, P.; et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell 2015, 27, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Ora, M.; Bathla, G.; Desai, A.; Gupta, V.; Agarwal, A. Ependymal Tumors: Overview of the Recent World Health Organization Histopathologic and Genetic Updates with an Imaging Characteristic. AJNR Am. J. Neuroradiol. 2024, 45, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Bertero, L.; Ricci, A.A.; Tampieri, C.; Cassoni, P.; Modena, P. Ependymomas. Pathologica 2022, 114, 436–446. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Santi, M.; Viaene, A.N.; Hawkins, C. Ependymal Tumors. Pediatr. Dev. Pathol. 2022, 25, 59–67. [Google Scholar] [CrossRef]
- Ellison, D.W.; Aldape, K.D.; Capper, D.; Fouladi, M.; Gilbert, M.R.; Gilbertson, R.J.; Hawkins, C.; Merchant, T.E.; Pajtler, K.; Venneti, S.; et al. cIMPACT-NOW update 7: Advancing the molecular classification of ependymal tumors. Brain Pathol. 2020, 30, 863–866. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Larrew, T.; Saway, B.F.; Lowe, S.R.; Olar, A. Molecular Classification and Therapeutic Targets in Ependymoma. Cancers 2021, 13, 6218. [Google Scholar] [CrossRef]
- Hübner, J.M.; Kool, M.; Pfister, S.M.; Pajtler, K.W. Epidemiology, molecular classification and WHO grading of ependymoma. J. Neurosurg. Sci. 2018, 62, 46–50. [Google Scholar] [CrossRef]
- Ozawa, T.; Kaneko, S.; Szulzewsky, F.; Qiao, Z.; Takadera, M.; Narita, Y.; Kondo, T.; Holland, E.C.; Hamamoto, R.; Ichimura, K. C11orf95-RELA fusion drives aberrant gene expression through the unique epigenetic regulation for ependymoma formation. Acta Neuropathol. Commun. 2021, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Mohankumar, K.M.; Punchihewa, C.; Weinlich, R.; Dalton, J.D.; Li, Y.; Lee, R.; Tatevossian, R.G.; Phoenix, T.N.; Thiruvenkatam, R.; et al. C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma. Nature 2014, 506, 451–455. [Google Scholar] [CrossRef]
- Kupp, R.; Ruff, L.; Terranova, S.; Nathan, E.; Ballereau, S.; Stark, R.; Sekhar Reddy Chilamakuri, C.; Hoffmann, N.; Wickham-Rahrmann, K.; Widdess, M.; et al. Translocations Constitute Ependymoma Chromatin Remodeling and Transcription Factors. Cancer Discov. 2021, 11, 2216–2229. [Google Scholar] [CrossRef]
- Lötsch, D.; Kirchhofer, D.; Englinger, B.; Jiang, L.; Okonechnikov, K.; Senfter, D.; Laemmerer, A.; Gabler, L.; Pirker, C.; Donson, A.M.; et al. Targeting fibroblast growth factor receptors to combat aggressive ependymoma. Acta Neuropathol. 2021, 142, 339–360. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Amoozgar, Z.; Uccello, T.P.; Lei, P.J.; Zhao, Y.; Ho, W.W.; Huang, P.; Kardian, A.; Mack, S.C.; Duda, D.G.; et al. Targeting EPHB2/ABL1 restores antitumor immunity in preclinical models of ependymoma. Proc. Natl. Acad. Sci. USA 2025, 122, e2319474122. [Google Scholar] [CrossRef]
- Donson, A.M.; Amani, V.; Warner, E.A.; Griesinger, A.M.; Witt, D.A.; Levy, J.M.M.; Hoffman, L.M.; Hankinson, T.C.; Handler, M.H.; Vibhakar, R.; et al. Identification of FDA-Approved Oncology Drugs with Selective Potency in High-Risk Childhood Ependymoma. Mol. Cancer Ther. 2018, 17, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Servidei, T.; Meco, D.; Trivieri, N.; Patriarca, V.; Vellone, V.G.; Zannoni, G.F.; Lamorte, G.; Pallini, R.; Riccardi, R. Effects of epidermal growth factor receptor blockade on ependymoma stem cells in vitro and in orthotopic mouse models. Int. J. Cancer 2012, 131, E791–E803. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Han, S.; Yan, C.; Yang, Y.; Li, Z.; Yang, Z. The role of clinical factors and immunocheckpoint molecules in the prognosis of patients with supratentorial extraventricular ependymoma: A single-center retrospective study. J. Cancer Res. Clin. Oncol. 2021, 147, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Andreiuolo, F.; Varlet, P.; Tauziède-Espariat, A.; Jünger, S.T.; Dörner, E.; Dreschmann, V.; Kuchelmeister, K.; Waha, A.; Haberler, C.; Slavc, I.; et al. Childhood supratentorial ependymomas with YAP1-MAMLD1 fusion: An entity with characteristic clinical, radiological, cytogenetic and histopathological features. Brain Pathol. 2019, 29, 205–216. [Google Scholar] [CrossRef]
- Eder, N.; Roncaroli, F.; Domart, M.C.; Horswell, S.; Andreiuolo, F.; Flynn, H.R.; Lopes, A.T.; Claxton, S.; Kilday, J.P.; Collinson, L.; et al. YAP1/TAZ drives ependymoma-like tumour formation in mice. Nat. Commun. 2020, 11, 2380. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Wei, Y.; Okonechnikov, K.; Silva, P.B.G.; Vouri, M.; Zhang, L.; Brabetz, S.; Sieber, L.; Gulley, M.; Mauermann, M.; et al. YAP1 subgroup supratentorial ependymoma requires TEAD and nuclear factor I-mediated transcriptional programmes for tumorigenesis. Nat. Commun. 2019, 10, 3914. [Google Scholar] [CrossRef]
- Georgescu, M.M.; Yell, P.; Mobley, B.C.; Shang, P.; Georgescu, T.; Wang, S.H.; Canoll, P.; Hatanpaa, K.J.; White, C.L.; Raisanen, J.M. NHERF1/EBP50 is an organizer of polarity structures and a diagnostic marker in ependymoma. Acta Neuropathol. Commun. 2015, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu-Chittenden, Y.; Huang, B.; Shim, J.S.; Chen, Q.; Lee, S.J.; Anders, R.A.; Liu, J.O.; Pan, D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012, 26, 1300–1305. [Google Scholar] [CrossRef]
- Oku, Y.; Nishiya, N.; Shito, T.; Yamamoto, R.; Yamamoto, Y.; Oyama, C.; Uehara, Y. Small molecules inhibiting the nuclear localization of YAP/TAZ for chemotherapeutics and chemosensitizers against breast cancers. FEBS Open Bio 2015, 5, 542–549. [Google Scholar] [CrossRef]
- Bayliss, J.; Mukherjee, P.; Lu, C.; Jain, S.U.; Chung, C.; Martinez, D.; Sabari, B.; Margol, A.S.; Panwalkar, P.; Parolia, A.; et al. Lowered H3K27me3 and DNA hypomethylation define poorly prognostic pediatric posterior fossa ependymomas. Sci. Transl. Med. 2016, 8, 366ra161. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Wen, J.; Sill, M.; Lin, T.; Orisme, W.; Tang, B.; Hübner, J.M.; Ramaswamy, V.; Jia, S.; Dalton, J.D.; et al. Molecular heterogeneity and CXorf67 alterations in posterior fossa group A (PFA) ependymomas. Acta Neuropathol. 2018, 136, 211–226. [Google Scholar] [CrossRef]
- Nambirajan, A.; Sharma, A.; Rajeshwari, M.; Boorgula, M.T.; Doddamani, R.; Garg, A.; Suri, V.; Sarkar, C.; Sharma, M.C. EZH2 inhibitory protein (EZHIP/Cxorf67) expression correlates strongly with H3K27me3 loss in posterior fossa ependymomas and is mutually exclusive with H3K27M mutations. Brain Tumor Pathol. 2021, 38, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Dyer, M.A.; Qadeer, Z.A.; Valle-Garcia, D.; Bernstein, E. ATRX and DAXX: Mechanisms and Mutations. Cold Spring Harb. Perspect. Med. 2017, 7, a026567. [Google Scholar] [CrossRef]
- Griesinger, A.M.; Josephson, R.J.; Donson, A.M.; Mulcahy Levy, J.M.; Amani, V.; Birks, D.K.; Hoffman, L.M.; Furtek, S.L.; Reigan, P.; Handler, M.H.; et al. Interleukin-6/STAT3 Pathway Signaling Drives an Inflammatory Phenotype in Group A Ependymoma. Cancer Immunol. Res. 2015, 3, 1165–1174. [Google Scholar] [CrossRef]
- Griesinger, A.M.; Witt, D.A.; Grob, S.T.; Georgio Westover, S.R.; Donson, A.M.; Sanford, B.; Mulcahy Levy, J.M.; Wong, R.; Moreira, D.C.; DeSisto, J.A.; et al. NF-κB upregulation through epigenetic silencing of LDOC1 drives tumor biology and specific immunophenotype in Group A ependymoma. Neuro-Oncology 2017, 19, 1350–1360. [Google Scholar] [CrossRef]
- Michealraj, K.A.; Kumar, S.A.; Kim, L.J.Y.; Cavalli, F.M.G.; Przelicki, D.; Wojcik, J.B.; Delaidelli, A.; Bajic, A.; Saulnier, O.; MacLeod, G.; et al. Metabolic Regulation of the Epigenome Drives Lethal Infantile Ependymoma. Cell 2020, 181, 1329–1345.e1324. [Google Scholar] [CrossRef]
- Witt, H.; Mack, S.C.; Ryzhova, M.; Bender, S.; Sill, M.; Isserlin, R.; Benner, A.; Hielscher, T.; Milde, T.; Remke, M.; et al. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell 2011, 20, 143–157. [Google Scholar] [CrossRef]
- Lin, G.L.; Wilson, K.M.; Ceribelli, M.; Stanton, B.Z.; Woo, P.J.; Kreimer, S.; Qin, E.Y.; Zhang, X.; Lennon, J.; Nagaraja, S.; et al. Therapeutic strategies for diffuse midline glioma from high-throughput combination drug screening. Sci. Transl. Med. 2019, 11, eaaw0064. [Google Scholar] [CrossRef]
- Jain, S.U.; Do, T.J.; Lund, P.J.; Rashoff, A.Q.; Diehl, K.L.; Cieslik, M.; Bajic, A.; Juretic, N.; Deshmukh, S.; Venneti, S.; et al. PFA ependymoma-associated protein EZHIP inhibits PRC2 activity through a H3 K27M-like mechanism. Nat. Commun. 2019, 10, 2146. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yu, M.; Bai, Y.; Yu, J.; Jin, F.; Li, C.; Zeng, R.; Peng, J.; Li, A.; Song, X.; et al. Elevated CXorf67 Expression in PFA Ependymomas Suppresses DNA Repair and Sensitizes to PARP Inhibitors. Cancer Cell 2020, 38, 844–856.e847. [Google Scholar] [CrossRef] [PubMed]
- Donovan, L.K.; Delaidelli, A.; Joseph, S.K.; Bielamowicz, K.; Fousek, K.; Holgado, B.L.; Manno, A.; Srikanthan, D.; Gad, A.Z.; Van Ommeren, R.; et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat. Med. 2020, 26, 720–731. [Google Scholar] [CrossRef]
- de Sousa, G.R.; Lira, R.C.P.; de Almeida Magalhães, T.; da Silva, K.R.; Nagano, L.F.P.; Saggioro, F.P.; Baroni, M.; Marie, S.K.N.; Oba-Shinjo, S.M.; Brandelise, S.; et al. A coordinated approach for the assessment of molecular subgroups in pediatric ependymomas using low-cost methods. J. Mol. Med. 2021, 99, 1101–1113. [Google Scholar] [CrossRef]
- Zhao, F.; Wu, T.; Wang, L.M.; Zhang, J.; Zhang, H.; Li, S.W.; Zhang, S.; Li, P.; Wang, B.; Luo, L.; et al. Survival and Prognostic Factors of Adult Intracranial Ependymoma: A Single-institutional Analysis of 236 Patients. Am. J. Surg. Pathol. 2021, 45, 979–987. [Google Scholar] [CrossRef]
- Mack, S.C.; Pajtler, K.W.; Chavez, L.; Okonechnikov, K.; Bertrand, K.C.; Wang, X.; Erkek, S.; Federation, A.; Song, A.; Lee, C.; et al. Therapeutic targeting of ependymoma as informed by oncogenic enhancer profiling. Nature 2018, 553, 101–105. [Google Scholar] [CrossRef]
- Abedalthagafi, M.S.; Wu, M.P.; Merrill, P.H.; Du, Z.; Woo, T.; Sheu, S.H.; Hurwitz, S.; Ligon, K.L.; Santagata, S. Decreased FOXJ1 expression and its ciliogenesis programme in aggressive ependymoma and choroid plexus tumours. J. Pathol. 2016, 238, 584–597. [Google Scholar] [CrossRef]
- Coluccia, A.; La Regina, G.; Naccarato, V.; Nalli, M.; Orlando, V.; Biagioni, S.; De Angelis, M.L.; Baiocchi, M.; Gautier, C.; Gianni, S.; et al. Drug Design and Synthesis of First in Class PDZ1 Targeting NHERF1 Inhibitors as Anticancer Agents. ACS Med. Chem. Lett. 2019, 10, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Patronas, N.J.; Courcoutsakis, N.; Bromley, C.M.; Katzman, G.L.; MacCollin, M.; Parry, D.M. Intramedullary and spinal canal tumors in patients with neurofibromatosis 2: MR imaging findings and correlation with genotype. Radiology 2001, 218, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, D.R.; Sill, M.; Okonechnikov, K.; Korshunov, A.; Yip, S.; Schutz, P.W.; Scheie, D.; Kruse, A.; Harter, P.N.; Kastelan, M.; et al. MYCN amplification drives an aggressive form of spinal ependymoma. Acta Neuropathol. 2019, 138, 1075–1089. [Google Scholar] [CrossRef]
- Scheil, S.; Brüderlein, S.; Eicker, M.; Herms, J.; Herold-Mende, C.; Steiner, H.H.; Barth, T.F.; Möller, P. Low frequency of chromosomal imbalances in anaplastic ependymomas as detected by comparative genomic hybridization. Brain Pathol. 2001, 11, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Raffeld, M.; Abdullaev, Z.; Pack, S.D.; Xi, L.; Nagaraj, S.; Briceno, N.; Vera, E.; Pittaluga, S.; Lopes Abath Neto, O.; Quezado, M.; et al. High level MYCN amplification and distinct methylation signature define an aggressive subtype of spinal cord ependymoma. Acta Neuropathol. Commun. 2020, 8, 101. [Google Scholar] [CrossRef]
- Stermann, A.; Huebener, N.; Seidel, D.; Fest, S.; Eschenburg, G.; Stauder, M.; Schramm, A.; Eggert, A.; Lode, H.N. Targeting of MYCN by means of DNA vaccination is effective against neuroblastoma in mice. Cancer Immunol. Immunother. 2015, 64, 1215–1227. [Google Scholar] [CrossRef]
- Bates, J.E.; Choi, G.; Milano, M.T. Myxopapillary ependymoma: A SEER analysis of epidemiology and outcomes. J. Neuro-Oncol. 2016, 129, 251–258. [Google Scholar] [CrossRef]
- Montero, A.S.; Tran, S.; Amelot, A.; Berriat, F.; Lot, G.; Gaillard, S.; Villa, C.; Polivka, M.; Adam, C.; Idbaih, A.; et al. Clinical characteristics and long-term surgical outcome of spinal myxopapillary ependymoma: A French cohort of 101 patients. J. Neuro-Oncol. 2021, 152, 491–499. [Google Scholar] [CrossRef]
- Witt, H.; Gramatzki, D.; Hentschel, B.; Pajtler, K.W.; Felsberg, J.; Schackert, G.; Löffler, M.; Capper, D.; Sahm, F.; Sill, M.; et al. DNA methylation-based classification of ependymomas in adulthood: Implications for diagnosis and treatment. Neuro-Oncology 2018, 20, 1616–1624. [Google Scholar] [CrossRef]
- Barton, V.N.; Donson, A.M.; Kleinschmidt-DeMasters, B.K.; Birks, D.K.; Handler, M.H.; Foreman, N.K. Unique molecular characteristics of pediatric myxopapillary ependymoma. Brain Pathol. 2010, 20, 560–570. [Google Scholar] [CrossRef]
- Ahmad, O.; Chapman, R.; Storer, L.C.; Luo, L.; Heath, P.R.; Resar, L.; Cohen, K.J.; Grundy, R.G.; Lourdusamy, A. Integrative molecular characterization of pediatric spinal ependymoma: The UK Children’s Cancer and Leukaemia Group study. Neurooncol. Adv. 2021, 3, vdab043. [Google Scholar] [CrossRef]
- Shimada, S.; Ishizawa, K.; Horiguchi, H.; Shimada, T.; Hirose, T. Subependymoma of the spinal cord and review of the literature. Pathol. Int. 2003, 53, 169–173. [Google Scholar] [CrossRef]
- Krishnan, S.S.; Panigrahi, M.; Pendyala, S.; Rao, S.I.; Varma, D.R. Cervical Subependymoma: A rare case report with possible histogenesis. J. Neurosci. Rural. Pract. 2012, 3, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Monoranu, C.M.; Huang, B.; Zangen, I.L.; Rutkowski, S.; Vince, G.H.; Gerber, N.U.; Puppe, B.; Roggendorf, W. Correlation between 6q25.3 deletion status and survival in pediatric intracranial ependymomas. Cancer Genet. Cytogenet. 2008, 182, 18–26. [Google Scholar] [CrossRef]
- Rajaram, V.; Gutmann, D.H.; Prasad, S.K.; Mansur, D.B.; Perry, A. Alterations of protein 4.1 family members in ependymomas: A study of 84 cases. Mod. Pathol. 2005, 18, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.S.; Doan, N.; Gelsomino, M.; Shabani, S. Intracranial Subependymoma: A SEER Analysis 2004–2013. World Neurosurg. 2017, 101, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Thierfelder, F.; Träger, M.; Soschinski, P.; Müther, M.; Edelmann, D.; Förster, A.; Geiler, C.; Kim, H.Y.; Filipski, K.; et al. TERT promoter mutation and chromosome 6 loss define a high-risk subtype of ependymoma evolving from posterior fossa subependymoma. Acta Neuropathol. 2021, 141, 959–970. [Google Scholar] [CrossRef]
- Rushing, E.J.; Cooper, P.B.; Quezado, M.; Begnami, M.; Crespo, A.; Smirniotopoulos, J.G.; Ecklund, J.; Olsen, C.; Santi, M. Subependymoma revisited: Clinicopathological evaluation of 83 cases. J. Neuro-Oncol. 2007, 85, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kweh, B.T.S.; Rosenfeld, J.V.; Hunn, M.; Tee, J.W. Tumor characteristics and surgical outcomes of intracranial subependymomas: A systematic review and meta-analysis. J. Neurosurg. 2022, 136, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Ren, X.; Zhang, J.; Jia, W. Clinical, radiological, and pathological features in 43 cases of intracranial subependymoma. J. Neurosurg. 2015, 122, 49–60. [Google Scholar] [CrossRef]
- Kong, L.Y.; Wei, J.; Haider, A.S.; Liebelt, B.D.; Ling, X.; Conrad, C.A.; Fuller, G.N.; Levine, N.B.; Priebe, W.; Sawaya, R.; et al. Therapeutic targets in subependymoma. J. Neuroimmunol. 2014, 277, 168–175. [Google Scholar] [CrossRef]
- Saleh, A.H.; Samuel, N.; Juraschka, K.; Saleh, M.H.; Taylor, M.D.; Fehlings, M.G. The biology of ependymomas and emerging novel therapies. Nat. Rev. Cancer 2022, 22, 208–222. [Google Scholar] [CrossRef]
- Bobola, M.S.; Jankowski, P.P.; Gross, M.E.; Schwartz, J.; Finn, L.S.; Blank, A.; Ellenbogen, R.G.; Silber, J.R. Apurinic/apyrimidinic endonuclease is inversely associated with response to radiotherapy in pediatric ependymoma. Int. J. Cancer 2011, 129, 2370–2379. [Google Scholar] [CrossRef][Green Version]
- Chamberlain, M.C.; Johnston, S.K. Temozolomide for recurrent intracranial supratentorial platinum-refractory ependymoma. Cancer 2009, 115, 4775–4782. [Google Scholar] [CrossRef]
- Rudà, R.; Bosa, C.; Magistrello, M.; Franchino, F.; Pellerino, A.; Fiano, V.; Trevisan, M.; Cassoni, P.; Soffietti, R. Temozolomide as salvage treatment for recurrent intracranial ependymomas of the adult: A retrospective study. Neuro-Oncology 2016, 18, 261–268. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Yuan, Y.; Wu, J.; Mendoza, T.; Vera, E.; Omuro, A.; Lieberman, F.; Robins, H.I.; Gerstner, E.R.; Wen, P.Y.; et al. A phase II study of dose-dense temozolomide and lapatinib for recurrent low-grade and anaplastic supratentorial, infratentorial, and spinal cord ependymoma. Neuro-Oncology 2021, 23, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Buccoliero, A.M.; Castiglione, F.; Rossi Degl’Innocenti, D.; Paglierani, M.; Maio, V.; Gheri, C.F.; Garbini, F.; Moncini, D.; Taddei, A.; Sardi, I.; et al. O6-Methylguanine-DNA-methyltransferase in recurring anaplastic ependymomas: PCR and immunohistochemistry. J. Chemother. 2008, 20, 263–268. [Google Scholar] [CrossRef]
- Meco, D.; Servidei, T.; Lamorte, G.; Binda, E.; Arena, V.; Riccardi, R. Ependymoma stem cells are highly sensitive to temozolomide in vitro and in orthotopic models. Neuro-Oncology 2014, 16, 1067–1077. [Google Scholar] [CrossRef]
- Sabnis, D.H.; Storer, L.C.D.; Liu, J.F.; Jackson, H.K.; Kilday, J.P.; Grundy, R.G.; Kerr, I.D.; Coyle, B. A role for ABCB1 in prognosis, invasion and drug resistance in ependymoma. Sci. Rep. 2019, 9, 10290. [Google Scholar] [CrossRef] [PubMed]
- Smith, A. EPEN-54. ACNS0831, phase III randomized trial of post-radiation chemotherapy in patients with newly diagnosed ependymoma ages 1 to 21 year. Neuro-Oncology 2020, 22, iii318–iii319. [Google Scholar] [CrossRef]
- CERN Foundation. CERN Clinical Trials. Available online: https://www.cern-foundation.org/education/clinical-trials/cern-clinical-trials (accessed on 26 March 2025).
- Ritzmann, T.A.; Chapman, R.J.; Kilday, J.P.; Thorp, N.; Modena, P.; Dineen, R.A.; Macarthur, D.; Mallucci, C.; Jaspan, T.; Pajtler, K.W.; et al. SIOP Ependymoma I: Final results, long-term follow-up, and molecular analysis of the trial cohort-A BIOMECA Consortium Study. Neuro-Oncology 2022, 24, 936–948. [Google Scholar] [CrossRef]
- Leblond, P.; Massimino, M.; English, M.; Ritzmann, T.A.; Gandola, L.; Calaminus, G.; Thomas, S.; Pérol, D.; Gautier, J.; Grundy, R.G.; et al. Toward Improved Diagnosis Accuracy and Treatment of Children, Adolescents, and Young Adults With Ependymoma: The International SIOP Ependymoma II Protocol. Front. Neurol. 2022, 13, 887544. [Google Scholar] [CrossRef]
- Horbinski, C.; Solomon, D.A.; Lukas, R.V.; Packer, R.J.; Brastianos, P.; Wen, P.Y.; Snuderl, M.; Berger, M.S.; Chang, S.; Fouladi, M.; et al. Molecular Testing for the World Health Organization Classification of Central Nervous System Tumors: A Review. JAMA Oncol. 2025, 11, 317–328. [Google Scholar] [CrossRef]
- Khatua, S.; Ramaswamy, V.; Bouffet, E. Current therapy and the evolving molecular landscape of paediatric ependymoma. Eur. J. Cancer 2017, 70, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.J.; Ghasemi, D.R.; Andreiuolo, F.; Zschernack, V.; Espariat, A.T.; Buttarelli, F.R.; Giangaspero, F.; Grill, J.; Haberler, C.; Paine, S.M.L.; et al. Optimizing biomarkers for accurate ependymoma diagnosis, prognostication, and stratification within International Clinical Trials: A BIOMECA study. Neuro-Oncology 2023, 25, 1871–1882. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steller, A.; Childress, A.; Koch, A.; Vallee, E.; Raskin, S. The Current Landscape of Molecular Pathology for the Diagnosis and Treatment of Ependymoma. J. Mol. Pathol. 2025, 6, 23. https://doi.org/10.3390/jmp6030023
Steller A, Childress A, Koch A, Vallee E, Raskin S. The Current Landscape of Molecular Pathology for the Diagnosis and Treatment of Ependymoma. Journal of Molecular Pathology. 2025; 6(3):23. https://doi.org/10.3390/jmp6030023
Chicago/Turabian StyleSteller, Alyssa, Ashley Childress, Alayna Koch, Emma Vallee, and Scott Raskin. 2025. "The Current Landscape of Molecular Pathology for the Diagnosis and Treatment of Ependymoma" Journal of Molecular Pathology 6, no. 3: 23. https://doi.org/10.3390/jmp6030023
APA StyleSteller, A., Childress, A., Koch, A., Vallee, E., & Raskin, S. (2025). The Current Landscape of Molecular Pathology for the Diagnosis and Treatment of Ependymoma. Journal of Molecular Pathology, 6(3), 23. https://doi.org/10.3390/jmp6030023







