Evaluation of a New Ethanol-Based Preservative Medium for Liquid-Based Cervical Cytology: A Performance Pilot Study for Molecular Applications
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Cell Lines
2.3. gDNA Extraction, Quality and Quantity DNA Evaluation
2.4. The Abbott Real-Time High-Risk HPV Test
2.5. Statistical Methods
3. Results
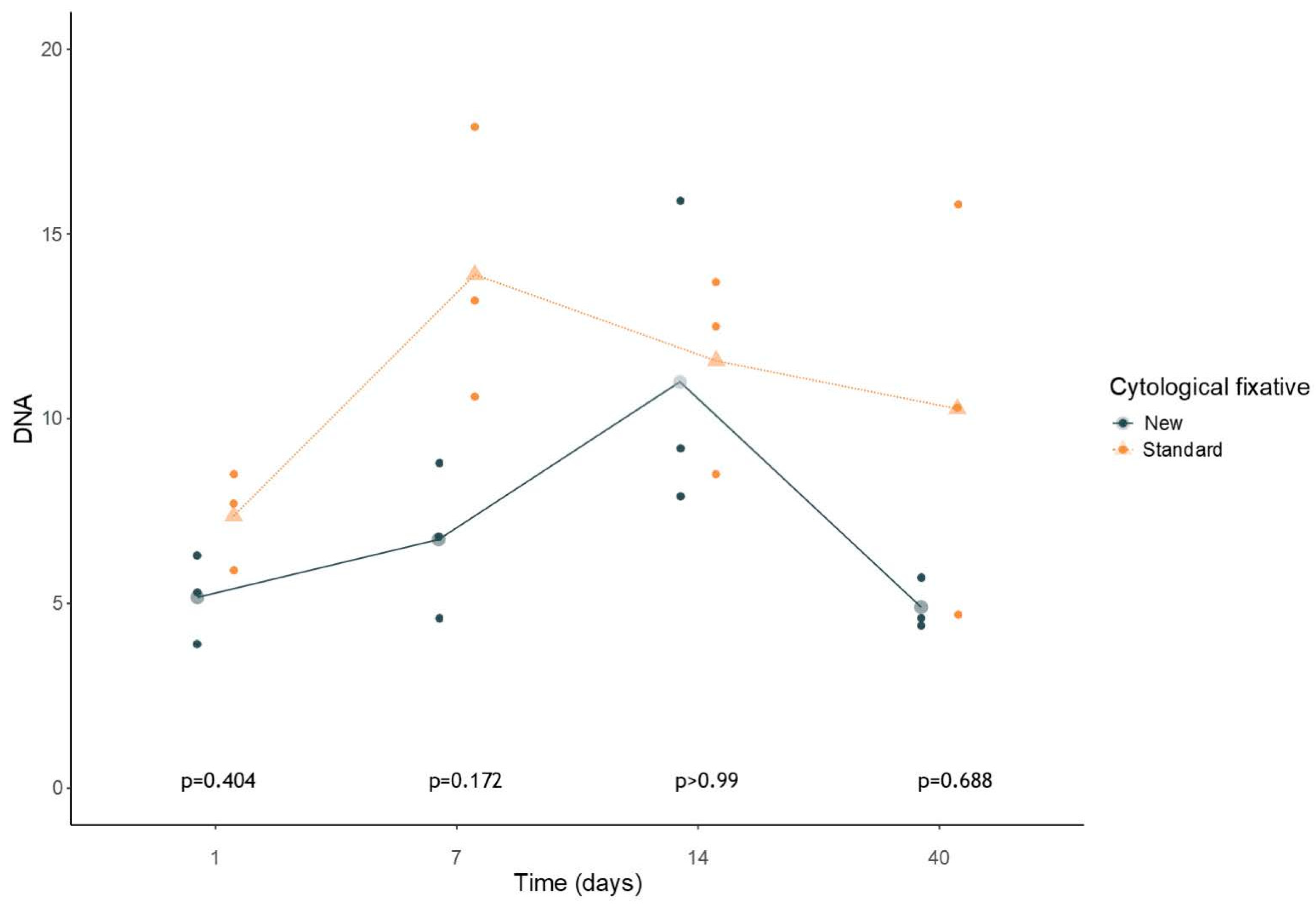
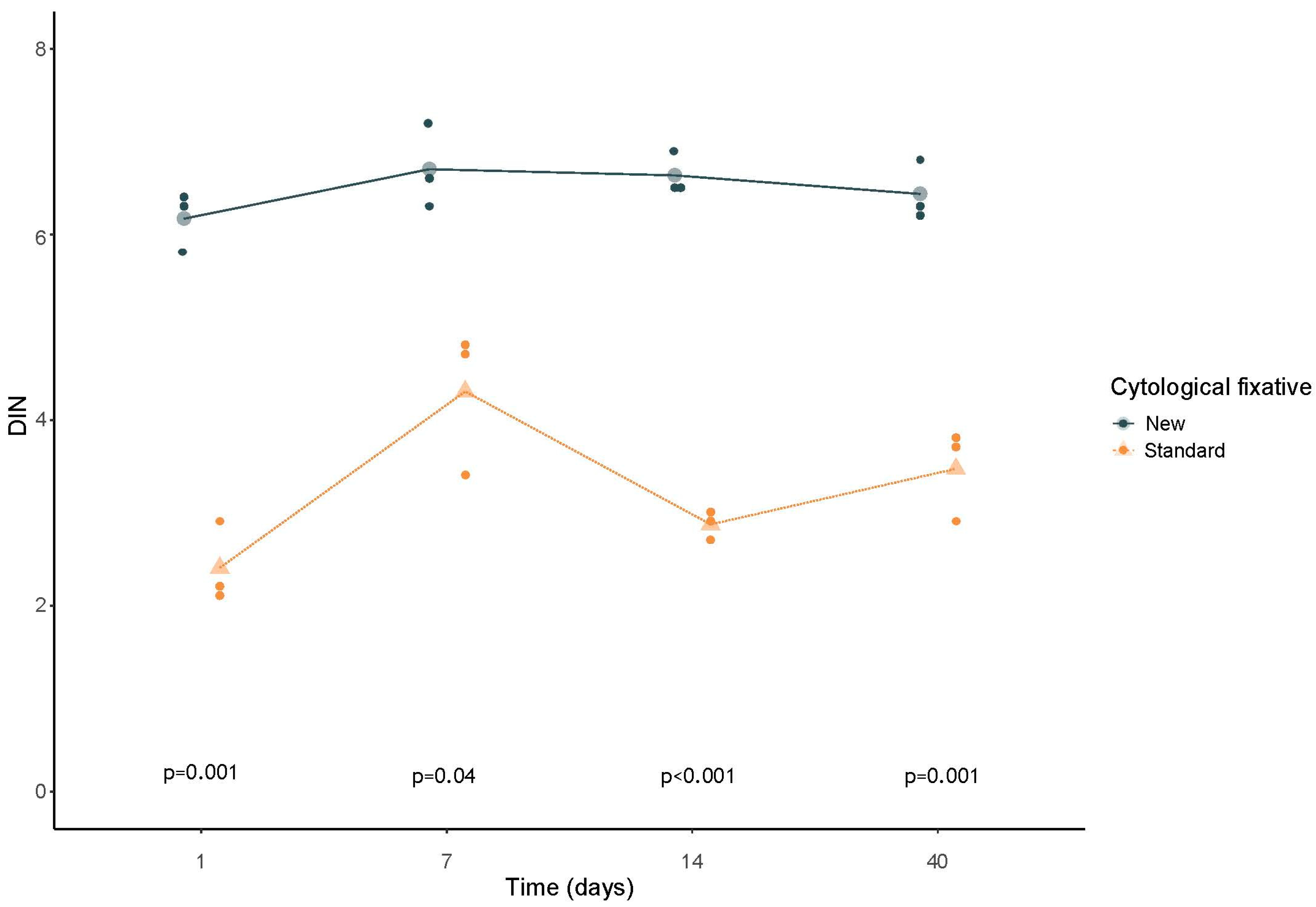
3.1. Quality and Quantity Genomic DNA Evaluation: Tape Station Cell Lines Samples Analysis
3.2. HPV Real-Time PCR Analysis in Cell Lines
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Institute NSW. HPV and Cervical Cancer; Borruto, F., De Ridder, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Parks, P. HPV; Reference Point Press: San Diego, CA, USA, 2015. [Google Scholar]
- Cancer Today. Available online: https://gco.iarc.fr/today/en (accessed on 20 March 2024).
- Nayar, R.; Wilbur, D.C. The Bethesda System for Reporting Cervical Cytology: A Historical Perspective. Acta Cytol. 2017, 61, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Pangarkar, M.A. The Bethesda System for Reporting Cervical Cytology. Cytojournal 2022, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Safaeian, M.; Solomon, D.; Castle, P.E. Cervical Cancer Prevention—Cervical Screening: Science in Evolution. Obs. Gynecol. Clin. N. Am. 2007, 34, 739. [Google Scholar] [CrossRef]
- Hoda, R.S.; Loukeris, K.; Abdul-Karim, F.W. Gynecologic Cytology on Conventional and Liquid-Based Preparations: A Comprehensive Review of Similarities and Differences. Diagn. CytoPathol. 2013, 41, 257–278. [Google Scholar] [CrossRef] [PubMed]
- Klinkhamer, P.J.J.M.; Meerding, W.J.; Rosier, P.F.W.M.; Hanselaar, A.G.J.M. Liquid-Based Cervical Cytology. Cancer CytoPathol. 2003, 99, 263–271. [Google Scholar] [CrossRef]
- Jeong, H.; Hong, S.R.; Chae, S.W.; Jin, S.Y.; Yoon, H.K.; Lee, J.; Kim, E.K.; Ha, S.T.; Kim, S.N.; Park, E.J.; et al. Comparison of Unsatisfactory Samples from Conventional Smear versus Liquid-Based Cytology in Uterine Cervical Cancer Screening Test. J. Pathol. Transl. Med. 2017, 51, 314–319. [Google Scholar] [CrossRef]
- Van Hemel, B.M.; Suurmeijer, A.J.H. Effective Application of the Methanol-Based PreservCyt(TM) Fixative and the Cellient(TM) Automated Cell Block Processor to Diagnostic CytoPathology, Immunocytochemistry, and Molecular Biology. Diagn. CytoPathol. 2013, 41, 734–741. [Google Scholar] [CrossRef]
- Perry, C.; Chung, J.Y.; Ylaya, K.; Choi, C.H.; Simpson, A.; Matsumoto, K.T.; Smith, W.A.; Hewitt, S.M. A Buffered Alcohol-Based Fixative for Histomorphologic and Molecular Applications. J. Histochem. Cytochem. 2016, 64, 425–440. [Google Scholar] [CrossRef]
- Bianchi, A.; Moret, F.; Desrues, J.M.; Champenois, T.; Dervaux, Y.; Desvouas, O.; Oursin, A.; Quinzat, D.; Dachez, R.; Bathelier, C.; et al. PreservCyt Transport Medium Used for the ThinPrep Pap Test Is a Suitable Medium for Detection of Chlamydia Trachomatis by the COBAS Amplicor CT/NG Test: Results of a Preliminary Study and Future Implications. J. Clin. Microbiol. 2002, 40, 1749–1754. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, Z.; Tian, Y.; Zhu, M.; Wang, S.; Wang, H.; Gao, S.; Ma, Y.; Zheng, M. Stability, Integrity, and Recovery Rate of Cellular Nucleic Acids Preserved in a New Liquid-Based Cytology Medium. Diagn. CytoPathol 2018, 46, 213–220. [Google Scholar] [CrossRef]
- Casatta, N.; Poli, A.; Bassani, S.; Veronesi, G.; Rossi, G.; Ferrari, C.; Lupo, C. Evaluation of a Novel Fixative Solution for Liquid-Based Cytology in Diagnostic CytoPathology. Diagnostics 2023, 13, 3601. [Google Scholar] [CrossRef]
- De Luna, R.; Eloubeidi, M.A.; Sheffield, M.V.; Eltoum, I.; Jhala, N.; Jhala, D.; Chen, V.K.; Chhieng, D.C. Comparison of ThinPrep and Conventional Preparations in Pancreatic Fine-Needle Aspiration Biopsy. Diagn. CytoPathol. 2004, 30, 71–76. [Google Scholar] [CrossRef]
- Chung, J.Y.; Song, J.S.; Ylaya, K.; Sears, J.D.; Choi, L.; Cho, H.; Rosenberg, A.Z.; Hewitt, S.M. Histomorphological and Molecular Assessments of the Fixation Times Comparing Formalin and Ethanol-Based Fixatives. J. Histochem. Cytochem. 2018, 66, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Clery, E.; Pisapia, P.; Migliatico, I.; Pepe, F.; De Luca, C.; Russo, M.; De Rosa, F.; Smeraglia, F.; Insabato, L.; Vigliar, E.; et al. Cytology Meets next Generation Sequencing and Liquid Biopsy: A Case of Lung Adenocarcinoma Presenting as Metastasis to the Phalanx. Diagn. CytoPathol 2020, 48, 759–764. [Google Scholar] [CrossRef]
- Pisapia, P.; Pepe, F.; Sgariglia, R.; Nacchio, M.; Russo, G.; Conticelli, F.; Girolami, I.; Eccher, A.; Bellevicine, C.; Vigliar, E.; et al. Next Generation Sequencing in Cytology. CytoPathology 2021, 32, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Agreda, P.M.; Beitman, G.H.; Gutierrez, E.C.; Harris, J.M.; Koch, K.R.; LaViers, W.D.; Leitch, S.V.; Maus, C.E.; McMillian, R.A.; Nussbaumer, W.A.; et al. Long-Term Stability of Human Genomic and Human Papillomavirus DNA Stored in BD SurePath and Hologic PreservCyt Liquid-Based Cytology Media. J. Clin. Microbiol. 2013, 51, 2702–2706. [Google Scholar] [CrossRef] [PubMed]
- ThinPrep® PreservCyt® Solution Instructions for Use. 2019. Available online: https://www.hologic.com/sites/default/files/2020-07/AW-19166-002_001_02.pdf (accessed on 7 May 2025).
- Huang, S.; Tang, N.; Mak, W.B.; Erickson, B.; Salituro, J.; Li, Y.; Krumpe, E.; Schneider, G.; Yu, H.; Robinson, J.; et al. Principles and Analytical Performance of Abbott RealTime High Risk HPV Test. J. Clin. Virol. 2009, 45, S13–S17. [Google Scholar] [CrossRef]
- Zhao, F.H.; Hu, S.Y.; Bian, J.J.; Liu, B.; Peck, R.B.; Bao, Y.P.; Pan, Q.J.; Frappart, L.; Sellors, J.; Qiao, Y.L. Comparison of ThinPrep and SurePath Liquid-Based Cytology and Subsequent Human Papillomavirus DNA Testing in China. Cancer CytoPathol. 2011, 119, 387–394. [Google Scholar] [CrossRef]
- von Karsa, L.; Arbyn, A.; Arbyn, M.; Anttila, A.; Jordan, J.; Ronco, G.; Schenck, U.; Segnan, N.; Wiener, H. Executive Summary. In European Guidelines for Quality Assurance in Cervical Cancer Screening; Publications Office of the European Union: Luxembourg, 2015; ISBN 9789279485398. [Google Scholar]
- Zhang, L.; Tan, W.; Yang, H.; Zhang, S.; Dai, Y. Detection of Host Cell Gene/HPV DNA Methylation Markers: A Promising Triage Approach for Cervical Cancer. Front. Oncol. 2022, 12, 831949. [Google Scholar] [CrossRef]
- Dey, P.; Luthra, U.A.C.K.; Path, F.R.C.; George, J.; Zuhairy, F.; George, S.S.; Haji, B.I. Comparison of ThinPrep and Conventional Preparations on Fine Needle Aspiration Cytology Material. Acta Cytol. 2000, 44, 46–50. [Google Scholar] [CrossRef]
- Matsuo, Y.; Yoshida, T.; Yamashita, K.; Satoh, Y. Reducing DNA Damage by Formaldehyde in Liquid-Based Cytology Preservation Solutions to Enable the Molecular Testing of Lung Cancer Specimens. Cancer CytoPathol. 2018, 126, 1011–1021. [Google Scholar] [CrossRef]

| Fissative Type | Diluition | Timing | CT |
|---|---|---|---|
| Preservcyt® | 1:5 | 1 day | 22.09 |
| 1:10 | 22.56 | ||
| 1:20 | 23.54 | ||
| Cytopath® | 1:5 | 22.71 | |
| 1:10 | 23.57 | ||
| 1:20 | 23.94 | ||
| Preservcyt® | 1:5 | 7 days | 21.63 |
| 1:10 | 23 | ||
| 1:20 | 23.96 | ||
| Cytopath® | 1:5 | 22.59 | |
| 1:10 | 22.35 | ||
| 1:20 | 23.91 | ||
| Preservcyt® | 1:5 | 14 days | 19.7 |
| 1:10 | 20.93 | ||
| 1:20 | 22.66 | ||
| Cytopath® | 1:5 | 21.55 | |
| 1:10 | 22.63 | ||
| 1:20 | 25.16 | ||
| Preservcyt® | 1:5 | 40 days | 20.47 |
| 1:10 | 22.09 | ||
| 1:20 | 22.85 | ||
| Cytopath® | 1:5 | 24.07 | |
| 1:10 | 23.79 | ||
| 1:20 | 22.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conticelli, F.; Pisapia, P.; Iaccarino, A.; Salatiello, M.; Venuta, A.; Gragnano, G.; Vallefuoco, L.; Sorrentino, R.; Portella, G.; Casatta, N.; et al. Evaluation of a New Ethanol-Based Preservative Medium for Liquid-Based Cervical Cytology: A Performance Pilot Study for Molecular Applications. J. Mol. Pathol. 2025, 6, 22. https://doi.org/10.3390/jmp6030022
Conticelli F, Pisapia P, Iaccarino A, Salatiello M, Venuta A, Gragnano G, Vallefuoco L, Sorrentino R, Portella G, Casatta N, et al. Evaluation of a New Ethanol-Based Preservative Medium for Liquid-Based Cervical Cytology: A Performance Pilot Study for Molecular Applications. Journal of Molecular Pathology. 2025; 6(3):22. https://doi.org/10.3390/jmp6030022
Chicago/Turabian StyleConticelli, Floriana, Pasquale Pisapia, Antonino Iaccarino, Maria Salatiello, Alessandro Venuta, Gianluca Gragnano, Luca Vallefuoco, Rosanna Sorrentino, Giuseppe Portella, Nadia Casatta, and et al. 2025. "Evaluation of a New Ethanol-Based Preservative Medium for Liquid-Based Cervical Cytology: A Performance Pilot Study for Molecular Applications" Journal of Molecular Pathology 6, no. 3: 22. https://doi.org/10.3390/jmp6030022
APA StyleConticelli, F., Pisapia, P., Iaccarino, A., Salatiello, M., Venuta, A., Gragnano, G., Vallefuoco, L., Sorrentino, R., Portella, G., Casatta, N., Lupo, C., Bruzzese, D., Troncone, G., & De Luca, C. (2025). Evaluation of a New Ethanol-Based Preservative Medium for Liquid-Based Cervical Cytology: A Performance Pilot Study for Molecular Applications. Journal of Molecular Pathology, 6(3), 22. https://doi.org/10.3390/jmp6030022











