Abstract
For molecular diagnostics of lung cancer samples, often only a small amount of material is available. The ever-increasing number of biomarker testing is in contrast to the amount of material obtained. In that case, cytological specimens, such as serous effusion samples, are one possible option. Effusion samples were prepared as sediment smears or cytospins or as a cell block if needed. Suitable tumor cells areas were marked by a cytopathologist and used for molecular diagnostics, including fast track analysis, parallel sequencing, and/or fluorescence in situ hybridization. In 62 cases of malignant effusion with cells of pulmonary adenocarcinoma, molecular diagnostics were carried out. A fast-track result with the high-resolution melting method for hotspot mutation of KRAS Exon 2 and EGFR exon 21 and fragment length analysis of EGFR exon 19 was available for 43 out of 47 samples (92%). Parallel sequencing was successful for 56 out of 60 samples (93.3%). In the same period, 108 FISH analyses were performed for MET amplification, followed by ROS1, RET, and ALK translocation analysis. If only a limited amount of tissue/biopsy is available, a malignant effusion is advisable to perform on the molecular diagnostics with a high success rate.
1. Introduction
Lung cancer is one of the most frequent malignancies and also one of the leading causes of death from a malignant disease worldwide [1]. In the course of the disease, serous effusions occur quite frequently, with pleural effusions being prevalent [2]. If cells of a pulmonary adenocarcinoma are found in a serous effusion, an advanced stage of the disease is diagnosed [3]. In these cases, rapid molecular diagnostics should be performed to search for driver mutations which might provide an option for targeted therapy. Approved drugs are available for the epidermal growth factor receptor (EGFR), proto-oncogene B-Raf (BRAF), mesenchymal–epithelial transition factor (MET) or Kirsten rat sarcoma viral oncogene homolog (KRAS) G12C mutations, anaplastic lymphoma kinase (ALK), RET proto-oncogene (rearranged during transfection; RET) and c-ros oncogene 1 (ROS1) translocations [4,5,6,7,8,9,10]. The molecular diagnostics were carried out with panel-based parallel sequencing (NGS) and covered all kinds of mutations, e.g., point mutations, deletions, and insertions [11,12,13]. Translocations, i.e., rearrangements of larger chromosome segments, and amplifications were very well identified by fluorescence in situ hybridization (FISH) analysis. Typical examples are the EML4::ALK translocation, ROS1 translocation and MET amplifications [14].
In this study, we performed a retrospective analysis of all cases of malignant effusion due to pulmonary adenocarcinoma, which were processed in our cytopathology lab in 2018 and 2019 and have undergone molecular diagnostics in our institution [15].
Far more molecular analyses of effusion preparations in cases of pulmonary adenocarcinoma have been performed than analyzed in this study. We have restricted this study to effusion samples that had been processed in-house to minimize variation in preanalytical steps as far as possible [16,17].
2. Materials and Methods
Effusion samples were sent to the lab in containers of various forms and sizes depending on the sample volume and the preferences of the clinical department. The samples were expected to be fresh and without any additives. After centrifugation, the technician decided whether sediment smears or cytospins were prepared. Routinely, four slides were prepared; one was immersed immediately into 96% ethanol for fixation, and the other three slides were left air-drying. The alcohol-fixed slide was used for the Papanicolaou stain, two of the air-dried slides were used for Hematoxylin and Eosin (H&E) stain and May-Gruenwald-Giemsa (MGG) stain, and the fourth slide was left for additional stains if needed. If immunochemistry was requested, a cell block was prepared following an in-house protocol based on “Gautinger Protokoll” [18].
If the final diagnosis of standard cytomorphology was malignant effusion in advanced pulmonary adenocarcinoma, comprehensive molecular diagnostics according to the national Network Genomic Medicine (nNGM) Lung Cancer were suggested to the attending clinician and offered to the patient. After obtaining written informed consent, molecular diagnostics were performed. Screening for ALK and for ROS1 rearrangement by immunochemistry was performed on sections of a cell block, as this is part of the fast-track analysis and our lung cancer routine diagnostics (results are not listed here). If positive, a confirming test by fluorescence in situ hybridization (FISH) was performed. If no cell block was available or cellularity was too low, ALK and ROS1 were analyzed by FISH using smears or cytospins (Supplementary Table S4).
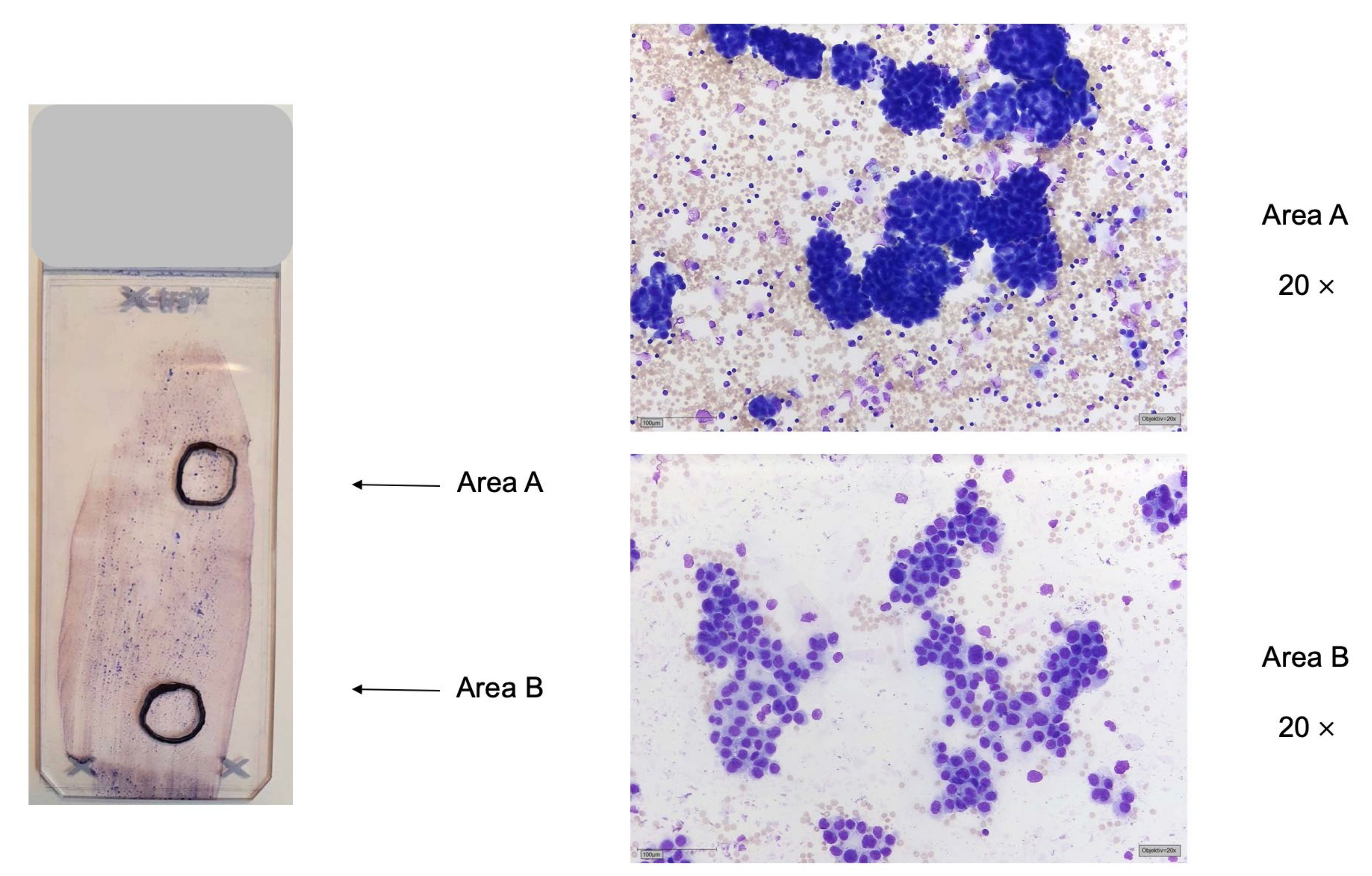
Since the result of the molecular diagnostics is of immediate clinical relevance for the choice of therapy, we perform a rapid analysis for EGFR exon 19 and 21 and KRAS exon 2 (also called “fast-track” analysis) with a very fast turnaround time. Analyses were carried out with high resolution melting or fragment length analysis, which detects a positive or negative result but does not provide the exact mutation description (Supplementary Method Description 1; Supplementary Table S1). Next-generation sequencing-based analysis of several genetic markers was performed using a panel approach. Validated gene panels with 14 genes using AmpliSeq gene panels for poor DNA quality or low DNA content samples (Thermo Fisher Scientific, Waltham, MA, USA; LUN3, 14 genes) or GeneRead gene panels for good DNA quality samples (Qiagen, Hilden, Germany; LUN5 panel) including 19 genes were used (Supplementary Method Description 2; Supplementary Tables S2 and S3 [19,20]). In many cases, a cell block of sufficient cellularity was available. In cases in which no cell block was available or the cellularity of the block was too low, stained sediment smears or cytospin preparations were used either for FISH analyses or for sequencing or for both assays. One of the cytopathologists reviewed the slides and decided which slides were used for the requested assays and marked the relevant areas on the glass slides (Figure 1).
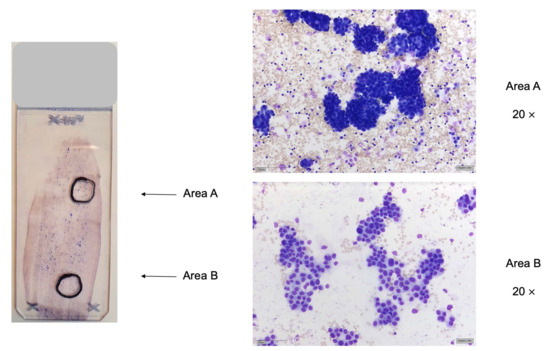
Figure 1.
Marking of suitable areas for DNA extraction (area (A)) or FISH analysis (area (B) by a cytopathologist. Left: sediment smear of pleural effusion of pulmonary adenocarcinoma, MGG stain. Two areas are marked up, area (A) for DNA extraction and parallel sequencing and area (B) for FISH analysis. Right: area (A): dense tumor cell clusters, suitable for DNA extraction and parallel sequencing; area (B): flat sheets of tumor cells, suitable for FISH analysis.
2.1. Preanalytics of Cytological Preparations
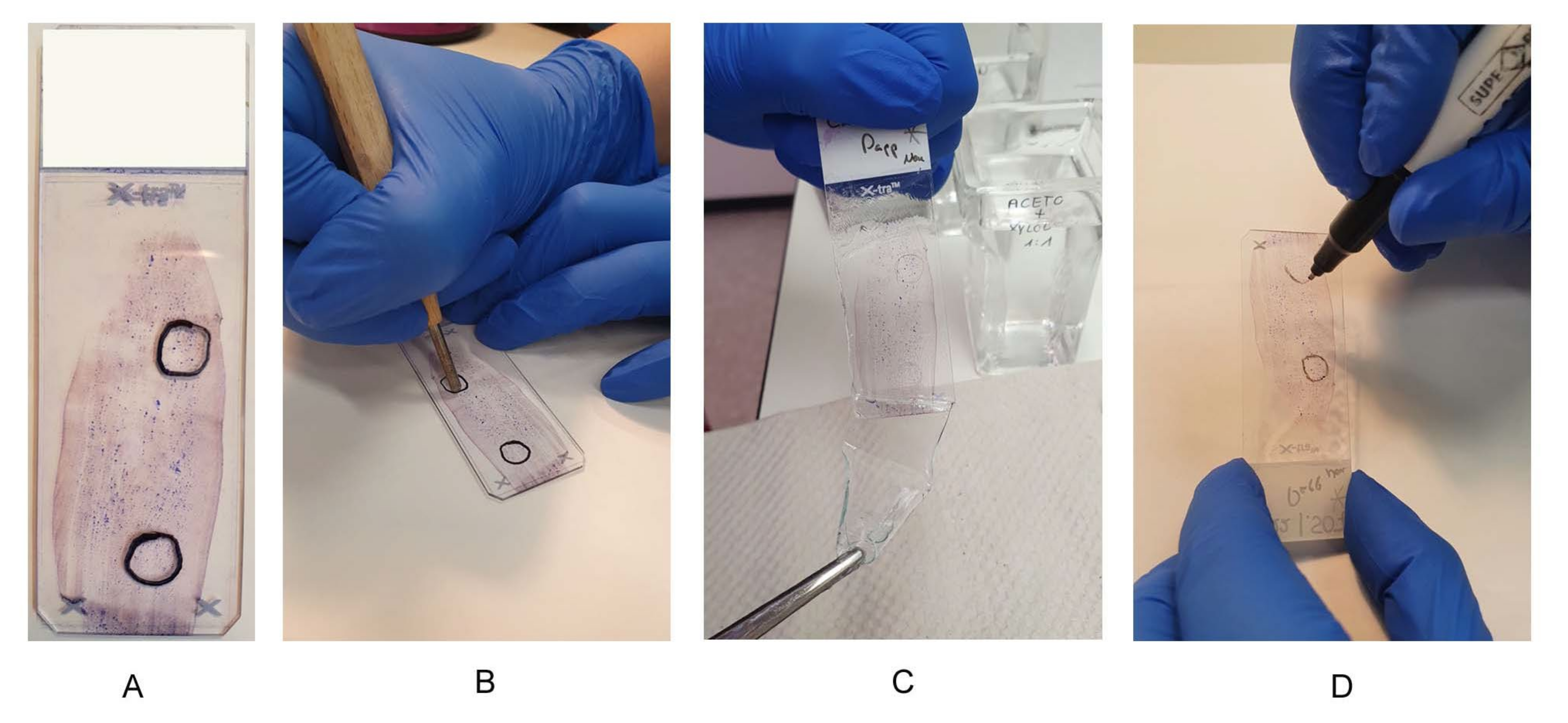
Cytological preparations can be sent in air-dried (not covered) or covered (coverslip or coverslip film) and already stained for analysis. In both cases, appropriate areas to be used for further analytical tests must be marked by an experienced cytopathologist (Figure 2A). For the uncovered specimen, the areas were marked on the back of the slide with a waterproof pen. Depending on how the cells were distributed, there may be one or more areas on a slide. If the marking was on the front side (coverslip or coverslip film), the marking was transferred to the back side of the slide. For all cytological preparations, the mark on the back of the slide was scratched into the glass with a diamond stylus (Figure 2B). This mark was then traced again with a waterproof pen. The smallest color pigments, which were not washed away by the xylene, were caught in the grooves (Figure 2D). These color traces and the traces of the diamond pen make it easier to find the marked area again afterwards.
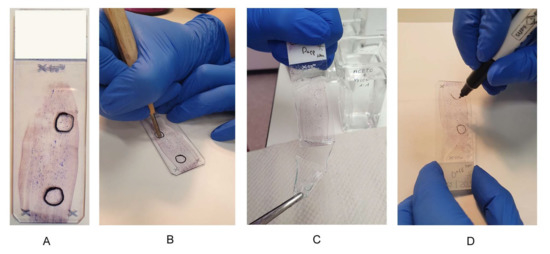
Figure 2.
Preanalytical steps on cytological preparations. Preanalytical steps on cytological preparations. (A) Suitable smear with two areas, one area for DNA extraction and one area for FISH analysis; (B) use of a diamond stylus, scratching the marking on the back of a slide; (C) uncovering the slide in xylene; (D) trace the diamond scratches with a waterproof pen.
An air-dried smear or cytospin was placed in water for one minute for DNA extraction. Subsequently, the marked area can be scraped off by macrodissection with a scalpel. The dissected material was transferred with the scalpel tip into a reaction vessel containing lysis buffer. The mixture was shaken overnight at 70 °C and further processed according to the manufacturer’s instructions. The extracted DNA was suitable for all subsequent PCR-based assays.
If the samples sent in were covered, the coverslip or coverslip film had to be removed first. A preparation that was covered with a coverslip was placed in xylene overnight. If the coverslip could not be removed the next morning, the smear or cytospin was placed in a −80 °C freezer for 30 min. The smear or cytospin was then placed back in xylene, and the coverslip should then be removable. If the submitted samples were covered with film, they were placed in acetone for 3.5 min, in an acetone/xylene mixture (1:2) for 1 min, and again in xylene for 1 min to completely remove the coverslip film (Figure 2C). It should be noted here that even markings made with xylene-resistant pens do not always adhere. The labeled area of the preparation was then “scraped off” with a scalpel and placed in a reaction tube filled with lysis buffer for DNA extraction.
2.2. Preparation for FISH Analysis
The cytological slides must be marked on the reverse side with a diamond pencil and then uncovered for FISH analysis. For FISH analysis, the smallest possible areas with at least 100 tumor cells that do not overlap were marked. If different FISH analyses have to be performed on one slide (smear or cytospin), the distance between the areas should be as large as possible. The pretreatment of the slides was automated using the VP2000 from Abbott and was identical to the pretreatment of formalin-fixed, paraffin-embedded (FFPE) sections. Slides that were already H&E, MGG, or Papanicolaou stained are well suited for FISH analysis. These preparations were decolorized by pretreating the slides for hybridization. After pretreatment, the 1st probe (3 µL) was applied and covered with a round coverslip (13 mm). The coverslip was sealed with Fixogum (Marabu). If the desired areas were close together, coverslips could be divided. After sealing the 1st area with Fixogum, the 2nd probe was applied to the 2nd area, covered, and sealed with Fixogum.
2.3. DNA Extraction from Cytological Preparations
For DNA extraction and subsequent PCR (polymerase chain reaction)-based analysis, at least one hundred malignant cells are required, which should be present “pure”, i.e., as little as possible mixed with benign epithelia and/or inflammatory cells. The tumor cell content should be at least 10% of the total cells for parallel sequencing. For other PCR-based assays, such as Sanger sequencing, the tumor cell content must be 20%, as this method is less sensitive. The percentage of tumor cells should be specified in order to be able to make a statement about an allelic fraction in case of a mutation detected by parallel sequencing. To increase the probability of mutation detection, it is useful not to extract all cells of a slide but to enrich the tumor cells in the number of cells that are extracted. Therefore, all available slides are stained (H&E) and reviewed microscopically. For DNA extraction, the cytopathologist marked a section of the preparation with a waterproof pen on the slide in which larger quantities of tumor cells are stored, if possible, without mixing with benign cells or inflammatory cells. Overlaying of the tumor cells themselves is not a problem (Figure 1).
Cytologic specimens may be air-dried or covered when labeled by the cytopathologist. Therefore, marking of the specimen may occur either on the coverslip or coverslip film or on the back of the slide. The mark was then transferred as described in the preanalytics section, see Figure 2. If a cell block was available from malignant effusions, molecular pathology studies were usually performed on the cell block. Marking of suitable areas was performed in the same way as on a biopsy. If necessary, additional sections, e.g., for FISH analysis, can be prepared. In our institution, the simultaneous sequencing of 14/19 genes and gene regions in a “lung cancer panel” using parallel sequencing is established as a routine method. The isolated DNA is measured for amplifiability and concentration by quantitative PCR (qPCR). If available, 10 ng of genomic DNA is used per primer pool. Since the “lung cancer panel” for parallel sequencing consists of four primer pools, a total of 40 ng of DNA must be used (Supplementary Table S1). According to the result of the parallel sequencing and depending on the request, different FISH analyses follow.
2.4. Fluorescence In Situ Hybridizations on Cytological Preparations
For FISH analysis, at least one hundred malignant cells are required in the preparation, which are well spread out (Figure 1). If possible, they should be without the overlay and well distinguishable from benign cells. An advantage of cytological preparations for FISH analysis is the intact cell nuclei, which are not partially incised as in a tissue preparation by cutting the FFPE blocks. However, the evaluation of FISH analyses can be more difficult because a whole-cell nucleus means that one has to look through the slide under fine focus. This diagnostic procedure is time-consuming but is worthwhile if a patient’s material is low. One problem with cytology slides is the residual stain from prestained slides, which cannot be completely destained during pretreatment for hybridization. Residues can elicit strong background autofluorescence so that the FISH analyses cannot be evaluated. For each FISH analysis, such an area in the preparation with one hundred well-spread, well-delineated cells is necessary (Figure 1). If sufficient suitable slides are available, a separate slide was used for each FISH examination. In the case of cell-rich, high-quality smears, several FISH analyses can be performed on the same slide (Figure 1). Especially with sediment smears of effusion fluid, this is often possible (Supplementary Method Description 3, [21,22,23,24,25].
3. Results
By searching the archived files, we identified 5702 samples of pleural effusions, ascites, and pericardial effusions, which were processed in the cytopathology lab of the Institute of Pathology of the University Hospital Cologne from January 2018 to December 2019. Of these, 288 cases with a final diagnosis of pulmonary adenocarcinoma with malignant effusion were identified. Among these cases were 256 pleural effusions, 15 ascites, and 17 pericardial effusions. For further analysis, the location of the effusion was not taken into account. In 62 cases of malignant effusion with cells of pulmonary adenocarcinoma, molecular diagnostics were performed. Of these, 40 cases were primary diagnoses of pulmonary adenocarcinoma in advanced stage. Cell blocks were available for molecular diagnostics in 53 out of 62 cases (Table 1).

Table 1.
Samples for molecular diagnostics.
Evaluation of Molecular Pathological Examinations of Cytological Specimens
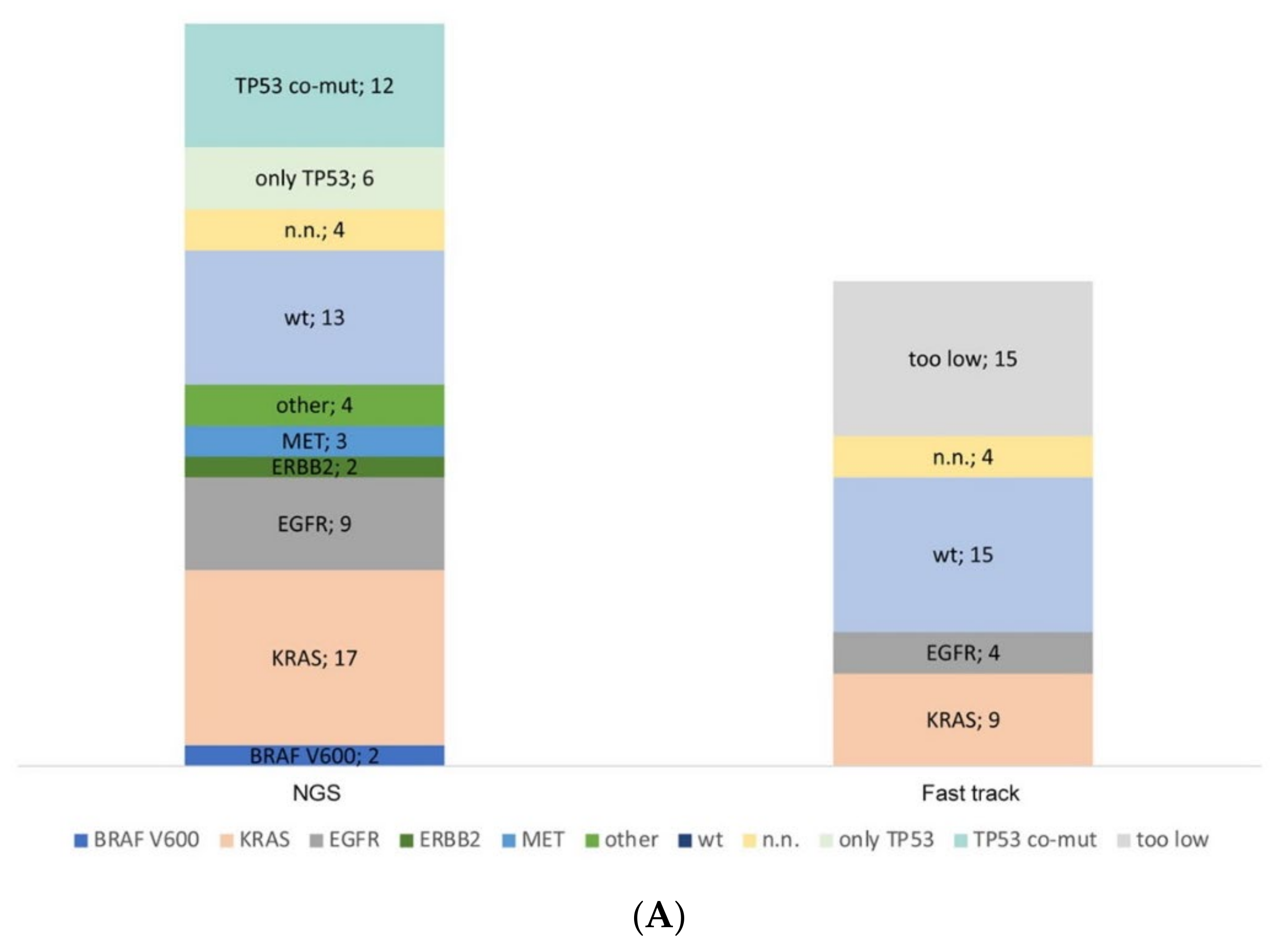
In the period between 2018 and 2019, 62 cytological lung cancer preparations (pleural effusion, pericardial effusion, ascites) from the pathology department of the University Hospital Cologne (in-house) were used for molecular diagnostics. Enough DNA for a fast-track analysis for hotspot mutation of KRAS Exon 2, EGFR exon 19 and 21 was available for 47 out of 62 samples (76%). Only four samples were not feasible for fast-track analysis, although they had a sufficient DNA concentration. A fast-track result was available for 92% (43 out of 47 samples). If DNA samples have a concentration of less than 5 ng/µL, the fast track is omitted, and only parallel sequencing is performed. In our study, 15 out of 62 samples did not reach this concentration limit (too low).
In 60 out of 62 samples of this series, parallel sequencing has been performed with a successful evaluation of 56 samples (93.3%, Figure 3A). Of the two remaining cases, in one of them, only fast track diagnostics had been performed before any further diagnostics had been cancelled because of clinical reasons. In the other case, only FISH analysis had been ordered by the clinician, as parallel sequencing was being performed simultaneously using a smear of a bronchial washing. As expected, most of the samples carried a KRAS mutation followed by TP53 as a co-occurring mutation. In 4 out of 60 samples, no amplicons could be obtained in parallel sequencing, and coverage was insufficient.
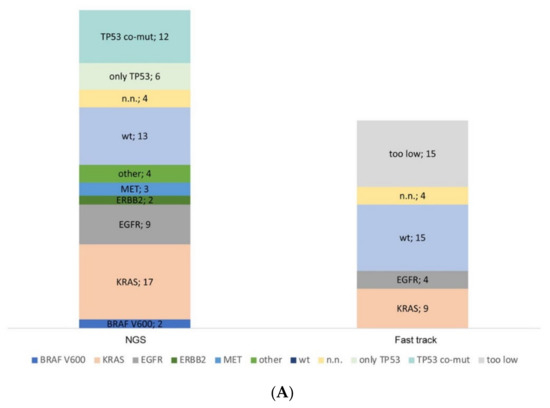
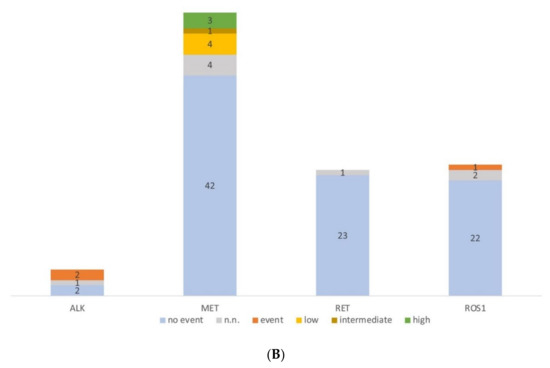
Figure 3.
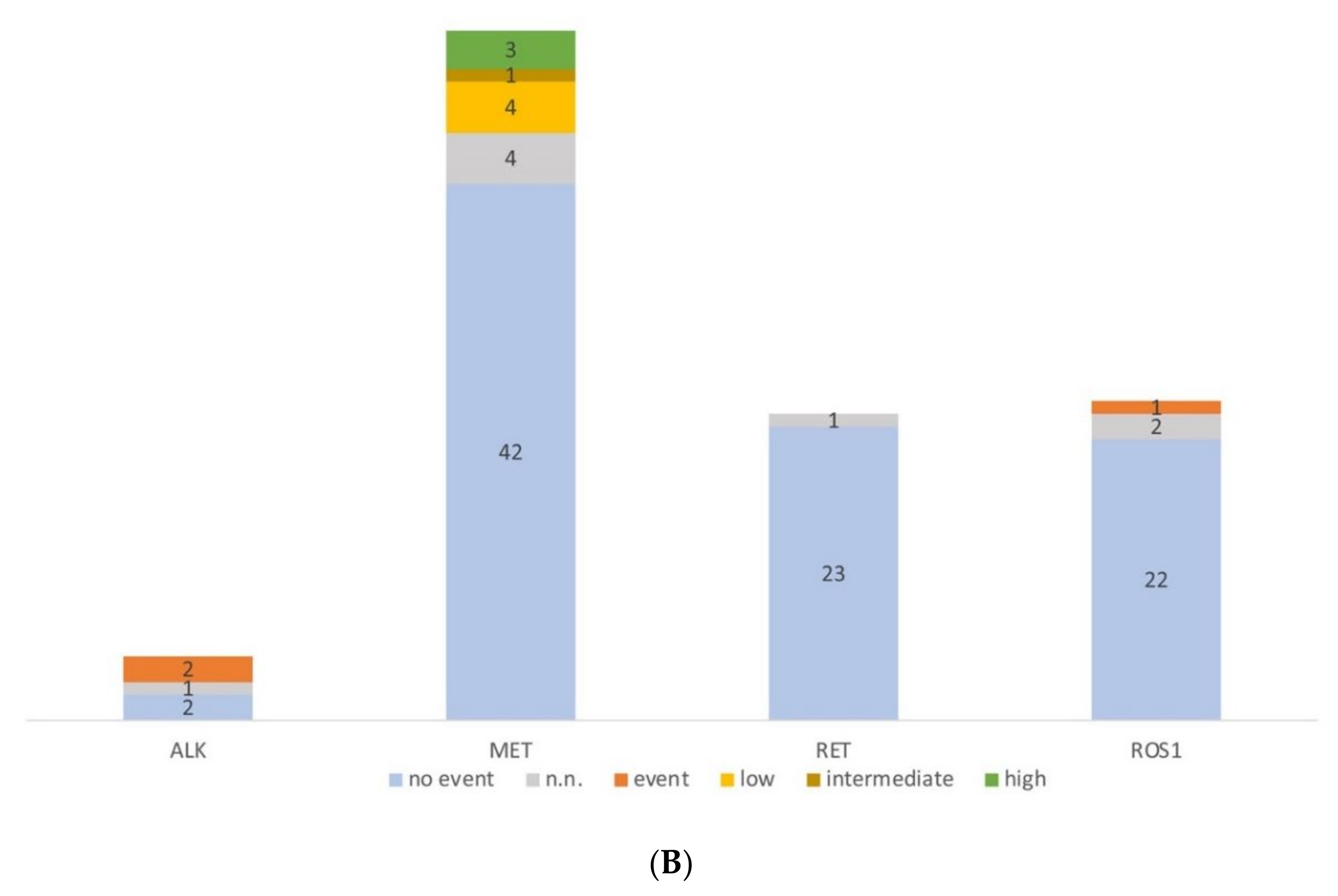
Molecular profiling of serous effusion samples with fast-track analysis, parallel sequencing and fluorescence in situ hybridization from 2018 to 2019. (A) Number of mutated and non-mutated samples for fast-track and parallel sequencing analysis. KRAS exon 2 and EGFR exon 19 and 21 within the fast track were analysed with high resolution melting and fragment length analysis. Too low: samples did not have >5 ng DNA/µL and were only analysed by parallel sequencing. N.n.: not detectable. In the parallel sequencing, 14/19 genes were analysed, of which the genes BRAF, KRAS, EGFR, ERBB2, MET, and TP53 were mutated most frequently; only TP53: the sample had no mutation other than a TP53 mutation; TP53 co-mut: the samples had an additional mutation to the TP53 mutation; other: the samples had a mutation outside the before mentioned genes. (B) Diagram of the number of fluorescence in situ hybridization on cytological preparations for ALK, RET, and ROS1 translocations and MET amplification. no event: no translocation or amplification, event: translocation or amplification, low/intermediate/high: low/intermediate/high amplification for MET. n.n.: not detectable.
In the same period, 108 FISH analyses were performed. The majority (n = 54) of FISH were MET amplification analysis (Figure 3B), followed by ROS1 (25), RET (24), and ALK translocation (5) analysis. We detected two ALK translocations (40%), three MET high-level amplifications (5.6%), and one ROS1 translocation (4%).
4. Discussion
Of a cohort of more than 5700 samples of pleural effusion, ascites, and pericardial effusion, which were processed in-house in 2018 and 2019, in 62 samples, molecular diagnostics for assessment of lung cancer were performed, 40 of these being primary diagnoses of pulmonary adenocarcinoma in advanced stage.
In 93.3% of these samples, parallel sequencing was successful. Fast-track testing for hotspot mutations of KRAS and EGFR gene was successful in a lesser proportion of 69.4% (43/62) of cases. Insufficient quantity of extracted DNA was the main reason for this draw-back. In cytology specimens, there is much more preanalytical variability than in histological specimen [26]. Different types of fixatives, sample recovery techniques from different companies, and previously stained slides are used, which increases the diversity of the samples. To our knowledge, there are no standardized preanalytical protocols for cytological specimens. Since optimizing protocols can be very challenging in everyday work, standardized protocols may discourage laboratories from continuing to perform their preanalytical methods according to long-established patterns. In this study, we could show a very high success rate for samples that were processed in-house due to our optimized preanalytical protocols. Reviewing the cytological slides and choosing adequate areas for DNA extraction and for FISH analysis is time-consuming but essential for the high success rates. In preanalytics, only small modifications are required for integrating cytological preparations into the workflow. The extracted DNA from these samples is suitable for all subsequent PCR-based assays. The evaluation of the sequencing data proceeded according to the quality criteria given in the Supplementary Materials. The cytological preparations used here all had at least a tumor cell content of 10%. Nevertheless, special care must be taken when evaluating the analyses: The variant table of parallel sequencing is evaluated at a very low tumor cell content, even below an allele frequency of 5%. Visual inspection reduces the risk of false-negative results [27].
As a limitation of the study, the molecular results from the effusion samples were not compared with a histological specimen from the same patient to evaluate any possible discordance in mutation or fusion detection. Thus, the possible risk of false negative or false positive findings in the cytological specimen was not investigated. We could show earlier that parallel sequencing on biopsy and smear material, respectively, had a complete concordance with the parallel sequencing results [28].
If already stained slides were used for molecular diagnostics and sacrificed, it is recommended that they must be digitally scanned before usage to record the most representative and diagnostic slide [26]. Other entities, such as serous high-grade carcinoma, also benefit from the investigation of the BRCA status on cytologic specimens [29]. This may result in reduced risks and costs associated with additional surgical biopsies and faster turnaround times, which in turn influence therapy options and clinical management.
FISH testing for ALK and ROS1 rearrangement has been decreasing in the last years due to the implementation of immunochemistry using cell block preparations for screening for ALK or ROS1 rearrangement. FISH is still required for testing for MET amplification and for RET translocation as well as for ALK and ROS1 translocation if no cell block is available or if immunochemistry screening is positive for one of the markers.
Other research groups have shown that ALK FISH on cell blocks compares favorably to immunohistochemistry and that cell blocks are not always absolutely necessary [30]. Smears and cytospins are equally suitable [31]. The result of immunohistochemistry is faster and cheaper to obtain in terms of probe and personnel costs compared to FISH. Therefore, cases are first stained by immunohistochemistry and FISH analysis is performed if positive. The same procedure applies to ROS1 translocations.
In the future, RNA-based fusion detection on cytological specimens will play an increasingly important role [32]. It is a good complement to DNA-based and FISH diagnostics as described here and is now standard on FFPE material [11,33]. In our experience, fusion detection on cytological specimens is challenging but not impossible. For RNA-based fusion detection, the already stained preparations are less suitable than the native preparations.
Testing rates for lung cancer patients in Germany are high and good by international comparison. Nevertheless, only three out of four patients are tested for alterations in EGFR, ALK, ROS1, and BRAF [34]. Cytological preparations may potentially help to increase the testing rate, as they are less invasive than biopsy or surgery.
We could show that DNA-based genomic profiling of lung adenocarcinoma is feasible using routine preparations of effusion samples and has a high success rate. Careful marking of cytological preparations contributes to the success of molecular pathological analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmp3020008/s1, Method Description 1: Fast Track Analysis, Method Description 2: Next Generation Sequencing, Method Description 3: fluorescence in situ hybridization, Table S1: Primer Design Fast Track Analysis for KRAS Exon 2 and EGFR exons 19 and 21, Table S2: Parallel sequencing panel design, Table S3: Primer design for parallel sequencing of lung cancer; Table S4: FISH probes for ALK, MET, RET and ROS1.
Author Contributions
Conceptualization, J.F. and M.E.; methodology, J.F.; formal analysis, J.F. and M.E.; resources, J.F. and M.E.; data curation, M.E.; writing—original draft preparation, J.F. and M.E.; writing—review and editing, R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the “Deutsche Krebshilfe”, funding number 70114428.
Institutional Review Board Statement
Not applicable. This study is restricted to retrospective data analysis of a cohort of effusion samples that were obtained as part of routine clinical care. No additional assays have been performed.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
Authors J.F. and M.E. declare no conflict of interest. R.B. has received honoraria for lectures and advisory boards from AbbVie, Amgen, AstraZeneca, Bayer, BMS, Boehringer-Ingelheim, Illumina, Janssen, Lilly, Merck-Serono, MSD, Novartis, Qiagen, Pfizer, Roche, and Targos MP Inc/DLS.
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, A.G.; Geisinger, K.; Aisner, S.C.; Al-Dayel, F.; Bubendorf, L. Terminology and criteria in non-resection specimens. In WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, 4th ed.; Travis, W.D., Brambilla, E., Burke, A.P., Marx, A., Nicholson, A.G., Eds.; International Agency for Research on Cancer (IARC): Lyon, France, 2015; pp. 17–21. [Google Scholar]
- Pisapia, P.; Pepe, F.; Iaccarino, A.; Sgariglia, R.; Nacchio, M.; Conticelli, F.; Salatiello, M.; Tufano, R.; Russo, G.; Gragnano, G.; et al. Next Generation Sequencing in Cytopathology: Focus on Non-Small Cell Lung Cancer. Front. Med. 2021, 8, 633923. [Google Scholar] [CrossRef]
- Passaro, A.; Mok, T.; Peters, S.; Popat, S.; Ahn, M.J.; de Marinis, F. Recent Advances on the Role of EGFR Tyrosine Kinase Inhibitors in the Management of NSCLC With Uncommon, Non Exon 20 Insertions, EGFR Mutations. J. Thorac. Oncol. 2021, 16, 764–773. [Google Scholar] [CrossRef]
- Cardarella, S.; Ogino, A.; Nishino, M.; Butaney, M.; Shen, J.; Lydon, C.; Yeap, B.Y.; Sholl, L.M.; Johnson, B.E.; Jänne, P.A. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin. Cancer Res. 2013, 19, 4532–4540. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Jain, P.; Wang, F.; Ma, P.C.; Borczuk, A.; Halmos, B. MET alterations and their impact on the future of non-small cell lung cancer (NSCLC) targeted therapies. Expert Opin. Ther. Targets 2021, 25, 249–268. [Google Scholar] [CrossRef]
- Liu, J.; Kang, R.; Tang, D. The KRAS-G12C inhibitor: Activity and resistance. Cancer Gene Ther. 2021. [Google Scholar] [CrossRef]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef]
- Mizukami, T.; Shiraishi, K.; Shimada, Y.; Ogiwara, H.; Tsuta, K.; Ichikawa, H.; Sakamoto, H.; Kato, M.; Shibata, T.; Nakano, T.; et al. Molecular mechanisms underlying oncogenic RET fusion in lung adenocarcinoma. J. Thorac. Oncol. 2014, 9, 622–630. [Google Scholar] [CrossRef] [Green Version]
- Pisapia, P.; Lozano, M.D.; Vigliar, E.; Bellevicine, C.; Pepe, F.; Malapelle, U.; Troncone, G. ALK and ROS1 testing on lung cancer cytologic samples: Perspectives. Cancer Cytopathol. 2017, 125, 817–830. [Google Scholar] [CrossRef] [Green Version]
- Heydt, C.; Wölwer, C.B.; Velazquez Camacho, O.; Wagener-Ryczek, S.; Pappesch, R.; Siemanowski, J.; Rehker, J.; Haller, F.; Agaimy, A.; Worm, K.; et al. Detection of gene fusions using targeted next-generation sequencing: A comparative evaluation. BMC Med. Genom. 2021, 14, 62. [Google Scholar] [CrossRef]
- Leichsenring, J.; Volckmar, A.L.; Kirchner, M.; Kazdal, D.; Kriegsmann, M.; Stögbauer, F.; Bockmayr, T.; Klauschen, F.; Herth, F.J.F.; Penzel, R.; et al. Targeted deep sequencing of effusion cytology samples is feasible, informs spatiotemporal tumor evolution, and has clinical and diagnostic utility. Genes Chromosomes Cancer 2018, 57, 70–79. [Google Scholar] [CrossRef]
- Roy-Chowdhuri, S.; Stewart, J. Preanalytic Variables in Cytology: Lessons Learned from Next-Generation Sequencing-The MD Anderson Experience. Arch. Pathol. Lab. Med. 2016, 140, 1191–1199. [Google Scholar] [CrossRef] [Green Version]
- Nambirajan, A.; Rana, D.; Samant, K.; Prabakaran, A.; Malik, P.; Jain, D. Multiplex fluorescence in situ hybridization testing for anaplastic lymphoma kinase and c-ros oncogene 1 gene rearrangements on cytology smears in lung adenocarcinomas: Comparative study with formalin-fixed paraffin-embedded sections. J. Am. Soc. Cytopathol. 2022, in press. [Google Scholar] [CrossRef]
- Huang, M.; Wei, S. Overview of Molecular Testing of Cytology Specimens. Acta Cytol. 2020, 64, 136–146. [Google Scholar] [CrossRef] [PubMed]
- da Cunha Santos, G.; Saieg, M.A.; Troncone, G.; Zeppa, P. Cytological preparations for molecular analysis: A review of technical procedures, advantages and limitations for referring samples for testing. Cytopathology 2018, 29, 125–132. [Google Scholar] [CrossRef]
- Bellevicine, C.; Malapelle, U.; Vigliar, E.; Pisapia, P.; Vita, G.; Troncone, G. How to prepare cytological samples for molecular testing. J. Clin. Pathol. 2017, 70, 819–826. [Google Scholar] [CrossRef]
- Herth, F.J.; Bubendorf, L.; Gütz, S.; Morresi-Hauf, A.; Hummel, M.; Junker, K.; Lehmann, U.; Petersen, I.; Schnabel, P.A.; Warth, A. Diagnose und prädiktive Analysen an zytologischen und bioptischen Tumorproben nicht-kleinzelliger Lungenkarzinome: Aktuelle Strategien und Herausforderungen [Diagnostic and predictive analyses of cytological specimens of non-small cell lung cancer: Strategies and challenges]. Pneumologie 2013, 67, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Michels, S.; Heydt, C.; van Veggel, B.M.; Deschler-Baier, B.; Pardo, N.; Monkhorst, K.; Rüsseler, V.; Stratmann, J.; Griesinger, J.; Steinhauser, S.; et al. Genomic Profiling Identifies Outcome-Relevant Mechanisms of Innate and Acquired Resistance to Third-Generation Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy in Lung Cancer. JCO Precis. Oncol. 2019, 3, 1–14. [Google Scholar] [CrossRef]
- Peifer, M.; Fernández-Cuesta, L.; Sos, M.L.; George, J.; Seidel, D.; Kasper, L.H.; Plenker, D.; Leenders, F.; Sun, R.; Zander, T.; et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat. Genet. 2012, 44, 1104–1110. [Google Scholar] [CrossRef]
- Heydt, C.; Kostenko, A.; Merkelbach-Bruse, S.; Wolf, J.; Büttner, R. ALK evaluation in the world of multiplex testing: Network Genomic Medicine (NGM): The Cologne model for implementing personalised oncology. Ann. Oncol. 2016, 27 (Suppl. S3), iii25–iii34. [Google Scholar] [CrossRef]
- Michels, S.; Scheel, A.H.; Scheffler, M.; Schultheis, A.M.; Gautschi, O.; Aebersold, F.; Diebold, J.; Pall, G.; Rothschild, S.; Bubendorf, L.; et al. Clinicopathological Characteristics of RET Rearranged Lung Cancer in European Patients. J. Thorac. Oncol. 2016, 11, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffler, M.; Schultheis, A.; Teixido, C.; Michels, S.; Morales-Espinosa, D.; Viteri, S.; Hartmann, W.; Merkelbach-Bruse, S.; Fischer, R.; Schildhaus, H.-S.; et al. ROS1 rearrangements in lung adenocarcinoma: Prognostic impact, therapeutic options and genetic variability. Oncotarget 2015, 6, 10577–10585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. Arch. Pathol. Lab. Med. 2014, 138, 241–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schildhaus, H.U.; Schultheis, A.M.; Rüschoff, J.; Binot, E.; Merkelbach-Bruse, S.; Fassunke, J.; Schulte, W.; Ko, Y.-D.; Schlesinger, A.; Bos, M.; et al. MET amplification status in therapy-naive adeno- and squamous cell carcinomas of the lung. Clin. Cancer Res. 2015, 21, 907–915. [Google Scholar] [CrossRef] [Green Version]
- Roy-Chowdhuri, S.; Aisner, D.L.; Allen, T.C.; Beasley, M.B.; Borczuk, A.; Cagle, P.T.; Capelozzi, V.; Dacic, S.; da Cunha Santos, G.; Hariri, L.P.; et al. Biomarker testing in lung carcinoma cytology specimens: A perspective from members of the Pulmonary Pathology Society. Arch. Pathol. Lab. Med. 2016, 140, 1267–1272. [Google Scholar] [CrossRef] [Green Version]
- Pisapia, P.; Malapelle, U.; Roma, G.; Saddar, S.; Zheng, Q.; Pepe, F.; Bruzzese, D.; Vigliar, E.; Bellevicine, C.; Luthra, R.; et al. Molecular Cytopathology Meeting Group. Consistency and reproducibility of next-generation sequencing in cytopathology: A second worldwide ring trial study on improved cytological molecular reference specimens. Cancer Cytopathol. 2019, 127, 285–296. [Google Scholar] [CrossRef]
- Hagmeyer, L.; Fassunke, J.; Engels, M.; Treml, M.; Herkenrath, S.; Matthes, S.; Büttner, R.; Randerath, W. Bronchoscopic Brushing from Central Lung Cancer-Next Generation Sequencing Results are Reliable. Lung 2019, 197, 333–337. [Google Scholar] [CrossRef]
- Lou, S.K.; Grenier, S.; Care, M.; McCuaig, J.; Stockley, T.L.; Clarke, B.; Ruff, H.M.; Boerner, S.L. Validation of BRCA testing on cytologic samples of high-grade serous carcinoma. Cancer Cytopathol. 2021, 129, 907–913. [Google Scholar] [CrossRef]
- Wang, W.; Tang, Y.; Li, J.; Jiang, L.; Jiang, Y.; Su, X. Detection of ALK rearrangements in malignant pleural effusion cell blocks from patients with advanced non-small cell lung cancer: A comparison of Ventana immunohistochemistry and fluorescence in situ hybridization. Cancer Cytopathol. 2015, 123, 117–122. [Google Scholar] [CrossRef]
- Savic, S.; Bubendorf, L. Common Fluorescence in Situ Hybridization Applications in Cytology. Arch. Pathol. Lab. Med. 2016, 140, 1323–1330. [Google Scholar] [CrossRef] [Green Version]
- Malapelle, U.; Pepe, F.; Pisapia, P.; Altimari, A.; Bellevicine, C.; Brunnström, H.; Bruno, R.; Büttner, R.; Cirnes, L.; De Andrea, C.E.; et al. Reference standards for gene fusion molecular assays on cytological samples: An international validation study. J. Clin. Pathol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ramani, N.S.; Chen, H.; Broaddus, R.R.; Lazar, A.J.; Luthra, R.; Medeiros, L.J.; Patel, K.P.; Rashid, A.; Routbort, M.J.; Stewart, J.; et al. Utilization of cytology smears improves success rates of RNA-based next-generation sequencing gene fusion assays for clinically relevant predictive biomarkers. Cancer Cytopathol. 2021, 129, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, F.; Eberhardt, W.; Nusch, A.; Reiser, M.; Zahn, M.O.; Maintz, C.; Bernhardt, C.; Losem, C.; Stenzinger, A.; Heukamp, L.C.; et al. CRISP Registry Group. Biomarker testing in non-small cell lung cancer in routine care: Analysis of the first 3,717 patients in the German prospective, observational, nation-wide CRISP Registry (AIO-TRK-0315). Lung Cancer 2021, 152, 174–184, Erratum in Lung Cancer 2021, 157, 167. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).