1. Introduction
Mixtures of collectors are widely used in the flotation of sulfide minerals and are believed to have performance benefits. An increase in valuable metal recovery, an increase in flotation kinetics and a reduction in reagent consumption has been reported [
1,
2,
3]. A fundamental study on the synergistic effect of mixtures of dithiocarbonates with different radical groups and dithiocarbonates mixed with xanthates on pyrite flotation was conducted by Bradshaw and O’Connor [
1], who observed that these thiol collector mixtures resulted in higher recoveries and grades than single thiol collectors. This was attributed to better surface coverage with mixed thiol collectors and, therefore, better hydrophobization. Thiol collectors such as dithiophosphates (DTP) and xanthates are said to have the ability to increase hydrophobicity and are also classified as good froth stabilizing agents [
1,
2,
3,
4]. These attributes of most thiol collectors result in recoveries of heterogeneous minerals such as base metal sulfides and may also result in high solids recoveries of both fine and coarse interlocked particles owing to a possible increase in froth stability [
2,
4]. Roy et al. [
5] concluded that the xanthates are able to adsorb selectively on fine particles, with co-collectors such as dithiophosphates and hydroxamate playing an important role in adsorbing onto coarse particles. Additionally, McFadzean et al. [
3] showed that collector mixtures had a beneficial effect in recovering heterogeneous ores such as PGMs, where many different valuable minerals were targeted by a specific collector within the collector mixture.
However, most of these studies were conducted in fresh or normal process water without considering variations in inorganic electrolytes resulting from onsite process water recirculation; the presence of electrolytes in process water may impact these reported beneficial effects of collector mixtures. Fundamental studies on water quality effects on flotation have shown that increases in the ionic strength of process water and specific inorganic electrolytes in process water can result in higher water and solids recoveries and, most importantly, affecting mineral grades and recoveries [
6,
7,
8,
9]. Recently, in a study that considered a Cu-Ni-PGM ore, mixtures of isobutyl xanthate (SIBX) and sodium diethyl dithiophosphate (SEDTP) in increasing ionic strength of plant water, it was shown that an increase in the ionic strength of plant water increased water recoveries and, therefore, froth stability in parallel with SEDTP’s froth-stabilizing effect, thus suggesting an additive interaction on the froth-stabilization effect of increasing ionic strength and SEDTP ratio. The presence of cationic electrolytes such as Ca
2+ and Mg
2+ has been found to have the ability to enhance froth stability; these electrolytes are known to decrease repulsive forces between collector ions and mineral surface, thereby enhancing bubble–particle attachment and mineral recoveries [
10,
11,
12,
13,
14]. This may result from the exothermic heat evolved when aqueous reagents are added, indicating that there is an interaction with negative thiol collector ions, which causes differences in the dilution enthalpies because of the attraction that occurs between the ions and water [
15]. However, other researchers have found that the collector adsorption may be hindered by metal ions that hydrolyze in alkaline conditions [
16,
17].
The literature also suggests that inorganic electrolytes can improve bubble–particle attachment efficiency through the compression of the electrical double layer, which, in turn, causes a reduction in the electrostatic repulsion between particles and bubbles and, therefore, results in increased floatability [
11]. It has been postulated that xanthate adsorbs onto the mineral surface via charge transfer between the collector and the mineral’s surface, while dithiophosphates adsorb via the formation of metal thiolates on the mineral surface [
18,
19]. These absorption reactions can, therefore, be affected by the presence of ions that are regarded as either structure makers or structure breakers [
14].
There is a growing need to understand whether the presence of common inorganic electrolytes in process water affects the behavior of mixtures of thiol collectors during the flotation of Cu-Ni-PGM ores. Therefore, this study investigates the effect of ion type on the behavior and performance of thiol collector mixtures during the flotation of a sulfidic Cu-Ni-PGM ore using NaCl and CaCl2 in the presence of sodium isobutyl xanthate (SIBX) and sodium diethyl dithiophosphate (SEDTP).
2. Materials and Methods
A 1 kg amount of a Merensky ore sample was milled at 66% solids in single-salt solutions in order to obtain a grind of 60%, passing 75 µm for 13.5 min, as determined by the milling curve shown in [
4], for each experimental test. The milled sample was transferred into a 3 L Barker flotation cell. The volume of the cell was made up to generate 35% solids using single-salt solutions (for the solution being investigated). The cell was fitted with a variable-speed drive, and the pulp level was controlled manually. The impeller speed was set at 1200 rpm. An air flow rate of 7 L/min was maintained for all flotation experiments, and a constant froth height of 2 cm was sustained throughout. The cell height was constantly corrected to 2 cm by the addition of the single-salt solution being tested. Collectors were dosed into the mill and, therefore, had a conditioning time of 13.5 min. The depressant and frother were dosed into the flotation cell and were allowed to condition for 2 min and 1 min, respectively.
Table 1 shows the suite of reagents used. Concentrates were collected at 1, 3, 7 and 20 min, respectively, by scraping the froth into concentrate trays every 15 s. A feed sample was taken before flotation and a tail sample after each flotation test. Feeds, concentrates and tails were filtered, dried in an oven, and weighed before Cu, Ni and S analyses.
A total collector dosage of 150 g/t of the selected thiol collector mixtures was used, and these were corrected for their active content or purity. The mixtures were based on the molar ratios shown in
Table 1. The characteristics of the chosen SIBX and SEDTP are shown in
Table 2. Moreover, 40 g/t of frother (Betachem modified DOW 200) was used throughout the flotation tests. The pH was kept constant at ~9.18. In order to induce the hydrophilicity of naturally floatable gangue (NFG) such as talc, a polysaccharide depressant used throughout the test work was Depramin 267 (modified CMC) at a dosage of 100 g/t. Reagents were supplied by Senmin, and the ore sample was obtained from the Bushveld Igneous Complex (BIC) in South Africa (SA). Two types of single salts were tested, namely sodium chloride (NaCl) and calcium chloride (CaCl
2), and these were of analytical grade.
Table 3 shows the chemistry, total dissolved solids (TDS) and the ionic strength (IS) of the single-salt solutions tested throughout this study. Each experimental test was repeated twice to minimise error. Statistical analyses were carried out, allowing for the determination of the standard error. The error bars shown in
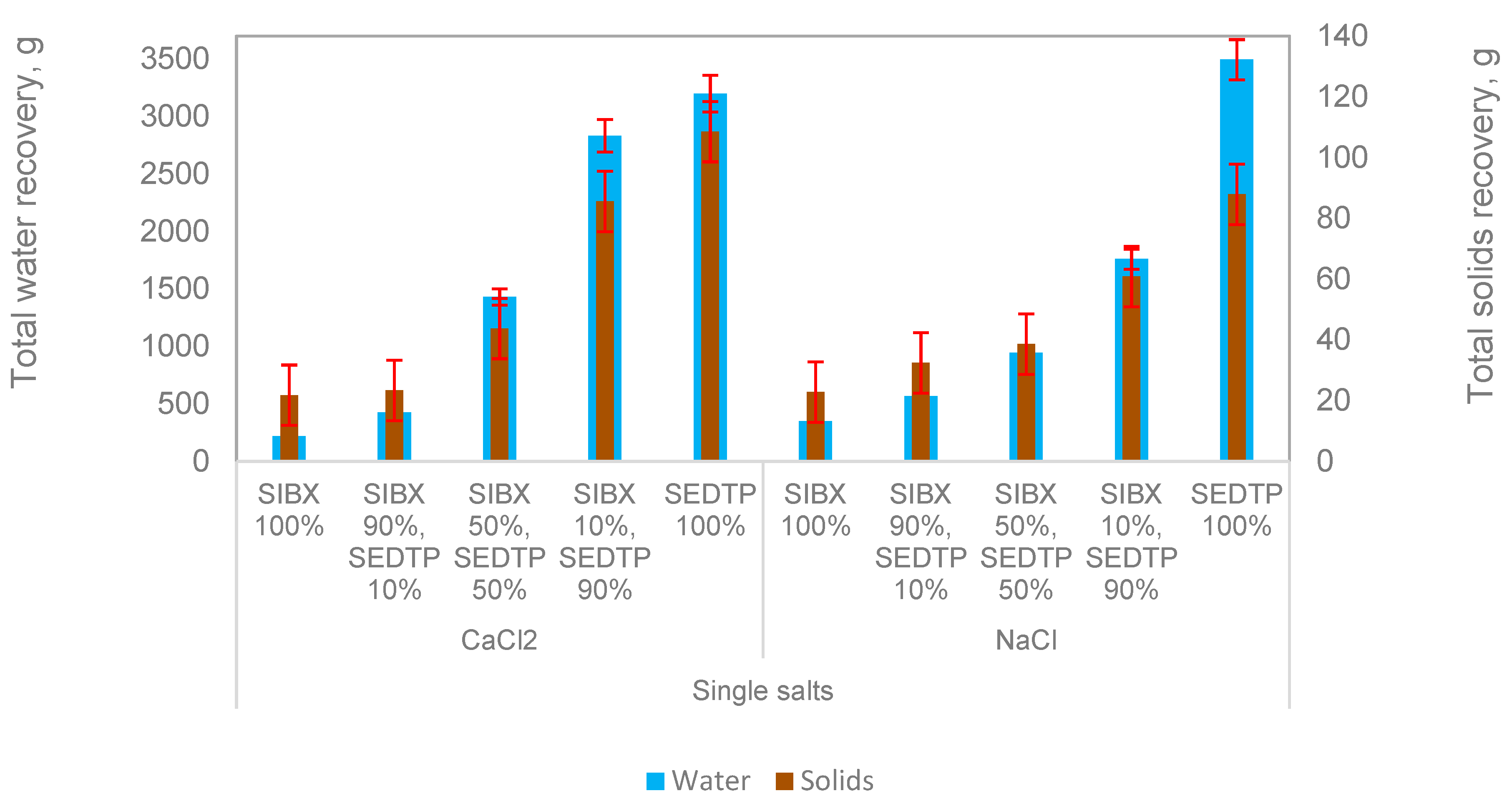
Figure 1,
Figure 2 and
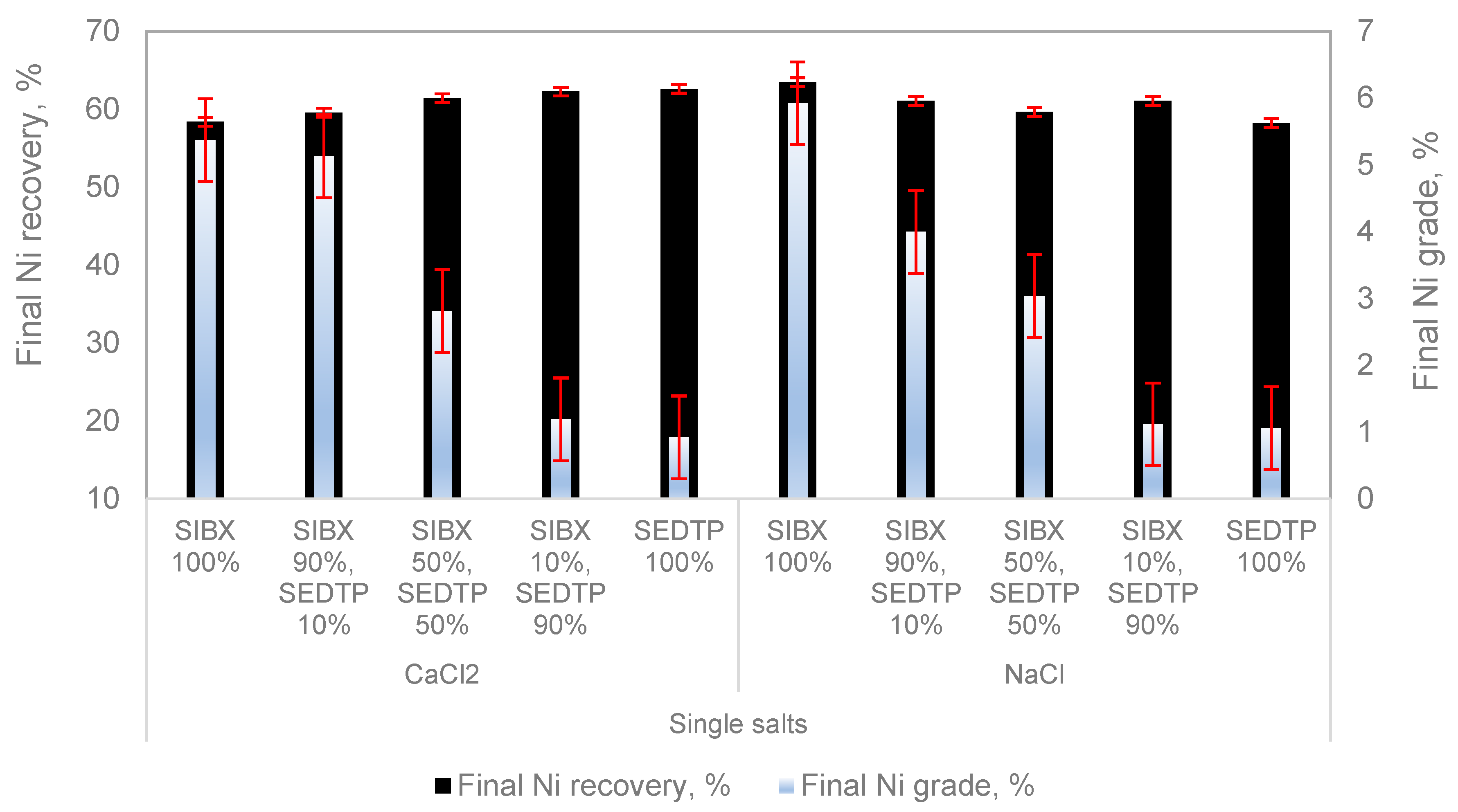
Figure 3 represent the standard error calculated from the replicate experimental runs.
3. Results and Discussion
Figure 1 shows the total cumulative solids and water recoveries for all conditions tested. It is evident that both solids and water recoveries generally increased with an increase in the molar ratio of SEDTP for both single-salt solutions. A dramatic increase in both solids and water recoveries is seen with SEDTP ratios above or equal to 50%, with 100% SEDTP pulling the most water and solids to the concentrate compared to 100% SIBX. It can also be seen that at 100% and 90% SIBX molar ratios, CaCl
2 resulted in similar solids and water recoveries compared to NaCl, while CaCl
2 resulted in higher solids and water recoveries at a higher SEDTP molar ratio (≥50%) as compared to NaCl, except that at a 100% SEDTP molar ratio, NaCl pulled more water compared to CaCl
2.
As illustrated in
Figure 1, water and solids recoveries increased with an increase in the SEDTP collector molar ratio for both single salts. This increase can be attributed to the frothing effect that dithiophosphate collectors are known to have [
2,
4]. It was also shown that, for 50% SEDTP and 90% SEDTP molar ratios, the divalent cation Ca
2+ resulted in higher solids and water recoveries for all thiol collector mixtures when compared to the monovalent cation, Na
+. This finding is in agreement with the froth-stabilizing nature of polyvalent cations compared to monovalent cations, which are less stabilizing on the froth [
10,
11,
12,
13,
20,
21]. Although it was expected that CaCl
2 would result in higher solids and water recoveries as compared to NaCl for all thiol mixtures, at 100% SIBX and 10% SEDTP molar ratios, NaCl resulted in similar water and solids recoveries.
This similarity in the behavior of the thiol collector mixtures in two different salts could be due to a different mechanism than that of froth stabilization. It is expected that Ca2+ would be more froth stabilizing and, therefore, would result in higher solids and water recoveries than Na+. The results on solids and water recoveries can be attributed to either the froth stabilizing or destabilizing action of the selected inorganic electrolytes in parallel with the collector combinations’ action on hydrophobicity and froth stability. These mechanisms should be investigated further.
Figure 2 shows the total cumulative Cu recoveries and grades for all tested conditions. It is evident that there was an increase in Cu recoveries and a decrease in Cu grades with an increase in SEDTP molar ratios in both single salts. For 100% SIBX, 90% SIBX:10% SEDTP, 50% SIBX:50% SEDTP and 10% SIBX:90% SEDTP, CaCl
2 resulted in higher Cu recoveries compared to NaCl. CaCl
2 and NaCl resulted in similar Cu recoveries when using 100% SEDTP. A 100% SIBX molar ratio resulted in fairly similar Cu grades in both CaCl
2 and NaCl. However, at 90% SIBX:10% SEDTP, CaCl
2 resulted in higher Cu grades than NaCl, while flotation with 50%, 90% and 100% SEDTP molar ratios in NaCl also resulted in similar Cu grades to those obtained in CaCl
2.
Figure 3 shows the final Ni recoveries and grades for all tested conditions. It can be seen that increases in SEDTP molar ratios resulted in a decrease in Ni grades in both CaCl
2 and NaCl. For each SIBX:SEDTP molar ratio, Ni grades in CaCl
2 were similar to those obtained in NaCl except for the 90% SIBX:10% SEDTP, as the use of CaCl
2 resulted in higher Ni grades than NaCl. In CaCl
2, an increase in SEDTP results in a slight increase in Ni recoveries. However, in NaCl, a slight decrease in Ni recoveries is seen with increasing SEDTP molar ratio; the lowest recovery is at a 100% SEDTP molar ratio and is similar to the Ni recovery with 100% SIBX in CaCl
2. Therefore, in CaCl
2, the highest Ni recovery is obtained with 100% SEDTP, and this is fairly comparable with the highest Ni recovery in NaCl, which was obtained at 100% SIBX.
Figure 2 shows an increase in Cu recoveries upon an increase in the SEDTP molar ratio for both single salts. It is also shown that Ni recoveries follow a similar trend to that of Cu recoveries in CaCl
2. A trend of a decrease in Ni recoveries with increasing SEDTP is seen in NaCl. These results are in line with the observations on solids and water recoveries and in agreement with reported improvements in mineral recoveries when using thiol collector mixtures [
2]. Cu and Ni grades decreased with an increased SEDTP molar ratio in solutions of CaCl
2 and NaCl. This can be attributed to an enhancement in froth stability resulting in more gangue being pulled to the concentrate [
22,
23]. It is also shown that the divalent cation, Ca
2+, results in higher Cu recoveries at all collector mixtures as compared to the monovalent cation, Na
+. This illustrates that divalent cations can enhance the hydrophobicity of thiol collectors better than monovalent cations as indicated by high Cu and Ni recoveries.
The presence of divalent cation, Ca
2+, resulted in higher Cu grades at higher SIBX molar ratios compared to Na
+, which resulted in higher Cu and Ni grades at higher SEDTP molar ratios than Ca
2+ ion. These results could indicate that the presence of these inorganic electrolytes influences the mineral surface chemistry and floatability differently. The increase in Ni recoveries with low Ni grades in the presence of the divalent cation, Ca
2+, shown in
Figure 3 could very well emanate from how Ca
2+ interacts and impact the surface chemistry of pentlandite particles. This could have resulted in nickel middlings being pulled into the concentrate, increasing the froth stability at higher SEDTP molar ratios of 90 and 100%, as shown in
Figure 3. These findings are in agreement with Wiese et al. [
24,
25], who showed that increasing froth stability results in an increase in mineral recoveries but also with a decrease in mineral grades. It is also illustrated that Ca
2+ resulted in higher Cu and Ni recoveries and lower mineral grades at mixed thiol collector molar ratio of 50:50 compared to Na
+ as shown in
Figure 2 and
Figure 3.
A fundamental study by Wang et al. [
26] showed that divalent cations such as Mg
2+ and Ca
2+ are more effective than monovalent cations such as Na
+ and K
+ in reducing the viscosity and, therefore, increasing copper flotation due to a stronger compression of the electrical double layer. The higher mineral recoveries and lower mineral grades at molar ratios of 50% SIBX: 50% SEDTP and 10% SIBX: 90% SEDTP in the presence of Ca
2+ shown in
Figure 2 and
Figure 3 suggest that the presence of ions in process water could either enhance or reduce collector selectivity during flotation. This is in agreement with McFadzean et al. [
3], who showed that collector mixtures were able to recover heterogeneous ores such as PGMs, where many different valuable minerals could be targeted by a specific collector in the mixture. They also found that some collectors effect an optimum hydrophobicity on the particles, making them good froth stabilizers, which is in line with the lower mineral grades and higher recoveries. The decrease in mineral grades with an increase in the SEDTP molar ratio suggests that unwanted gangue minerals could have been pulled to the concentrates leading to higher solids recoveries. Further investigative work needs to be conducted in order to understand the underlying mechanisms of inorganic electrolytes on the adsorption of thiol collector mixtures.
4. Conclusions
Water and solids recoveries increased with an increase in the SEDTP collector molar ratio for both single salts. This increase was attributed to the frothing effect that dithiophosphate collectors are known to have. Ca2+ resulted in higher solids and water recoveries in thiol collector mixtures containing more than a 50% SEDTP molar ratio compared to Na+. However, Na+ resulted in higher water at a molar ratio of 100% SEDTP. This study showed an increase in Cu recoveries and a decrease in Cu grades in increasing SEDTP molar ratios in both single salts. The divalent cation, Ca2+, resulted in higher Cu and Ni recoveries at all collector mixtures than the monovalent cation, Na+, owing to the froth-stabilizing nature of Ca2+ and its possible effects on the hydrophobization of sulfide minerals. In CaCl2, the highest Ni recovery was obtained using a 100% SEDTP molar ratio, and this was fairly comparable with the highest Ni recovery in NaCl, which was obtained at a 100% SIBX molar ratio.
Ca2+ resulted in lower mineral grades at higher SEDTP molar ratios compared to Na+. Ca2+ may have aided thiol collector selectivity at a high SIBX molar ratio, but selectivity reduced with the increase in the SEDTP molar ratio. This should, however, be a topic for further investigative test work.
The decrease in mineral grades with an increase in the SEDTP molar ratio suggests that unwanted gangue minerals were pulled to the concentrate leading to higher solids recoveries. Further investigative work, however, needs to be conducted in order to understand the underlying mechanisms of inorganic electrolytes on the adsorption of thiol collector mixtures.
The results of this study have shown that specific ions in process water may affect the behavior of mixtures of thiol collectors differently depending on the chemistry of the ions and their interfacial effects on the pulp phase.