1. Introduction
Pigment is the term used to designate a solid, organic or inorganic particle, with specific colors (white and black, for example) or fluorescent, which is insoluble in the substrate in which it will be incorporated and also does not promote any reaction with it [
1,
2].
Annatto dye is a natural organic pigment belonging to carotenoids, being widely used as a food dye due to its low toxicity. This dye consists of two main components, which are bixin and norbixin [
3]. In addition, it is less stable and easily discolored, so that light is the most destructive agent [
4].
In this sense, the protection of the dye molecule by other materials, clays for example, is one of the approaches that has been gaining prominence in recent years. Clays are natural materials, basically composed of organic matter, magnesium and aluminum lamellar silicates, quartz, feldspars and metal oxides. They also have clay minerals in their composition and among them, montmorillonite, which belongs to the smectite group, stands out.
Thus, the main purpose of this project was to develop hybrid materials, using annatto dye and clay modified with inorganic cations, aiming at the incorporation of bixin in the synthetic clay montmorillonite, and later to evaluate the stability of the new hybrid pigments.
2. Methodology
2.1. Materials
The annatto dye (bixin) was obtained commercially at an open market in the city of Teresina-PI. Synthetic sodium montmorillonite clay, calcium nitrate tetrahydrate (Ca (NO3) 2 · 4H2O), purity 99%, MM = 236.15 g mol−1, aluminum acetate (CH3COO) 2Al (OH), purity 99%, MM = 162.08 g mol−1, magnesium acetate tetrahydrate ((CH3COO) 2Mg · 4H2O), purity 99%, MM = 214.45 g mol−1, iron (III) nitrate nonahydrate (FeN3O9 · 9H2O), purity 99%, MM = 404.00 g mol−1, copper nitrate trihydrate (Cu (NO3) 2 · 3H2O), purity 99%, Sigma Aldrich, MM = 241.60 g mol−1, and ethyl alcohol (ethanol) PA were obtained from Sigma-Aldrich and used without previous purification.
2.2. Methods
Sample preparation was carried out following the method proposed by Fournier et al. [
5]. The process involved the preparation of metal cation solutions. Subsequently, sodium montmorillonite clay was placed in the prepared solution, leaving it under stirring for a period of 24 h. Then, the material was centrifuged and washed, and placed again in another solution containing the same precursor salt, and the same conditions were maintained and the same procedures were repeated. Finally, there was drying in the oven at 50 °C for 24 h.
The adsorption of the dye in the modified clay minerals was carried out as follows: first, 0.225 g of the dye was dissolved in a solution of water and ethanol (1: 4 v/v), then filtered and mixed with 0.3 g of the modified clay mineral. The mixture was subjected to stirring for 4 h, then it was centrifuged, washed and dried in an oven at 50 °C for 24 h.
Subsequently, the samples were subjected to some characterizations: UV/visible spectrophotometry, spectroscopy in the Infrared Region with Fourier Transform and Colorimetric test.
3. Results and Discussion
3.1. UV/Visible Spectrophotometry
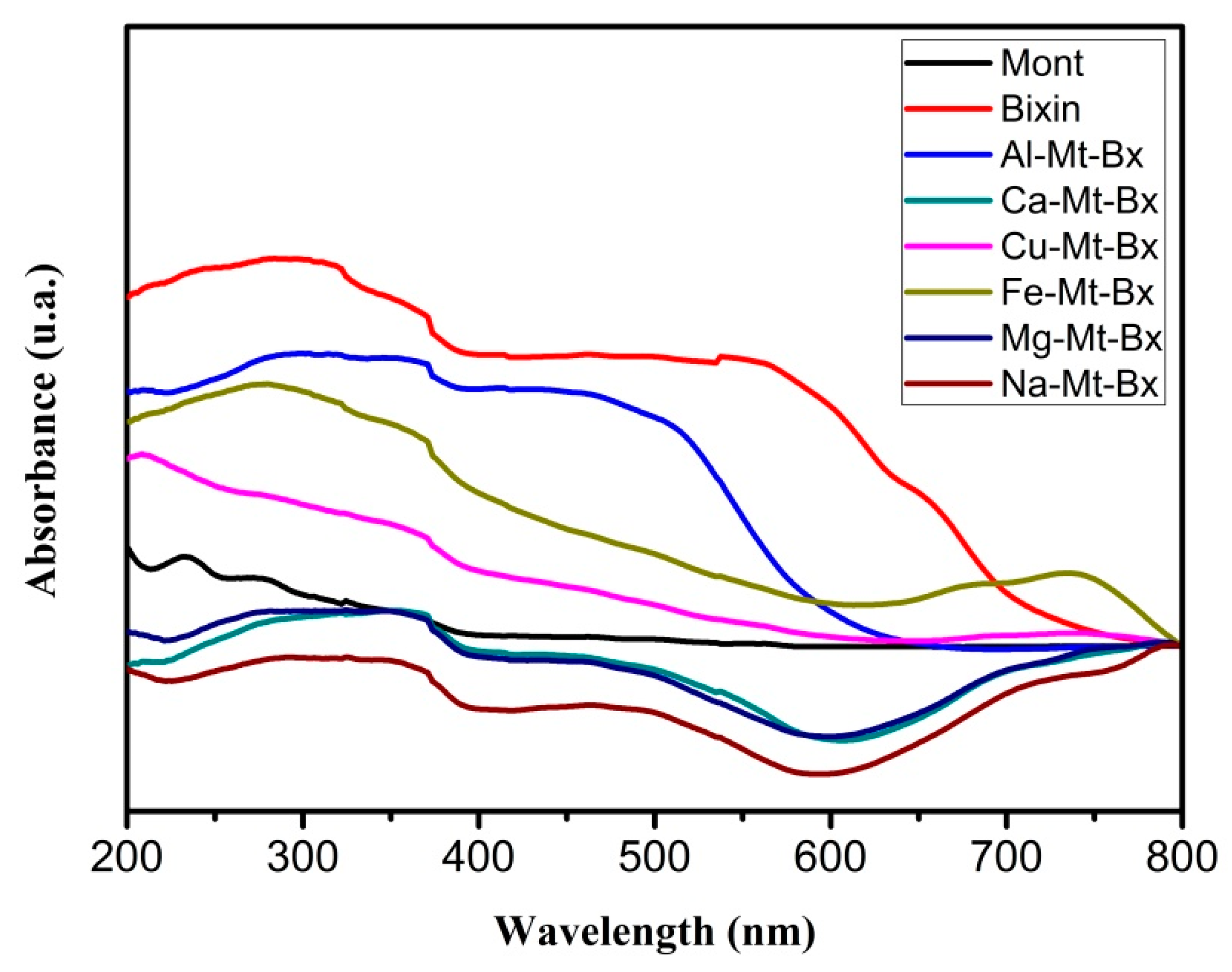
The UV/visible spectrophotometry (
Figure 1) for Na-Mt-Bx, Mg-Mt-Bx and Ca-Mt-Bx samples showed similar spectra, while another set of samples, Cu-Mt-Bx, Fe-Mt-Bx and Al-Mt-Bx, presented a behavior different from those and spectra similar to each other. In general, it is noticeable that none of the samples presented a spectrum similar to that of bixin, suggesting that there was a greater variation in colors in relation to all cations used. Thus, it is evident that the amount of adsorbed dye and the nature of the metallic cations in the space between layers modified the pigment structure, influencing the different colors of the hybrids [
6].
3.2. Infrared Spectroscopy with Fourier Transform
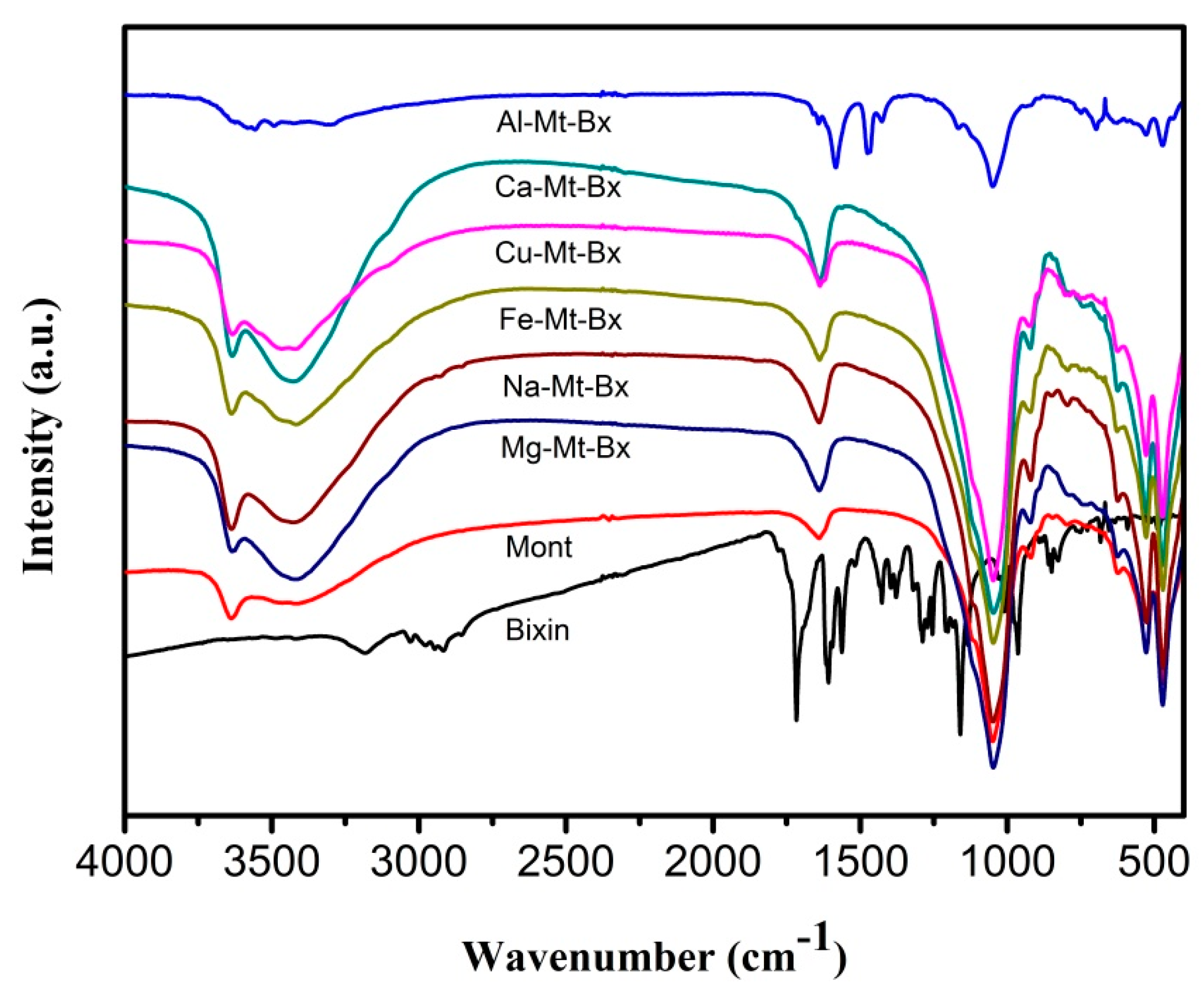
For clay mineral, the spectra can be divided into two regions: the region with a low wave number or deformations (1700 cm
−1 to 500 cm
−1); and the region of high wave numbers or stretching of those referring to the hydroxyl group Al-OH (3635 cm
−1) and to the hydration hydroxyl around 3390 cm
−1 [
7,
8].
The vibrational spectra of the hybrid materials (
Figure 2) showed similarities in their bands, with the OH bands initially being observed in the high frequency region, around 3638 cm
−1 attributed to the stretches of the Al-OH bond, and in 3440 cm
−1. The bands at 3444 cm
−1 and 1640 cm
−1 indicate the presence of stretching and deformation, respectively, of the hydroxyl group in the water. The bands in the region around 1640 and 922 cm
−1 were associated with angular deformations of the OH bonds of water and OH linked to the metallic cation of the octahedral sheet, in the case of aluminum [
9,
10].
3.3. Colorimetric Test
The colorimetric tests (
Figure 3) proved that the bixin showed a dark red color, with this color prevailing over the other colors in RGB, which is based on 100% red, 0% green and 0% blue, that is, (255,0,0) [
11]. Montmorillonite was white in color. In the RGB color model, it is compromised of 100% red, 100% green and 100% blue, which corresponds, in the cube, to (255,255,255). The hybrid materials Ca-Mt-Bx, Fe-Mt-Bx and Cu-Mt-Bx showed a color closer to light or dark brown. The Mg-Mt-Bx and Na-Mt-Bx samples had tones closer to gray and yellow. The hybrid pigment that showed the color that most differed from the others was the one that contained aluminum, as it had an orange tint.
4. Conclusions
The process of obtaining the hybrid pigments was successful. Observing the reflectance curves, the amount of dye adsorbed and the nature of the metallic cations in the space between layers modified the structure of the pigments, influencing the difference, as to the color of the hybrids.
The FTIR spectra showed that the characteristic bands of the dye were more noticeable when it was adsorbed on the mineral clay modified with aluminum, and through the colorimetric test, a variety of colors were found, according to the type of cation used.
Therefore, the hybrid pigment consisting of bixin and montmorillonite modified with Al3+ cations was the one that stood out the most, presenting improved properties when compared to the dye in its pure form. It is believed that, in the future, it may be used in the most diverse industrial areas, mainly in cosmetics and pharmaceuticals, where it is gaining more and more prominence.
Author Contributions
A.F.d.A.O.: Methodology, Investigation, Writing—original draft; P.T.: Methodology, Investigation and Visualization; D.H.L.D.: Investigation; L.M.C.H.: Investigation; J.A.O.: Supervision and Visualization and E.C.S.-F.: Supervision, Conceptualization, Project administration, Writing—review & editing.
Funding
This research was funded by CAPES and CNPq agencies, thank for the financial support and study/research grants.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lacerda, C.M. Elementary Characterization and Analysis of the Superficial Electrical Resistivity of Pigments Used in Tattoos. Master’s Thesis, Federal Technological University of Paraná, Curitiba, Brazil, 2017. [Google Scholar]
- Guillermin, D.; Debroise, T.; Trigueiro, P.; de Viguerie, L.; Rigaud, B.; Morlet-savary, F.; Balme, S.; Janot, J.M.; Tielens, F.; Michot, L.; et al. New pigments based on carminic acid and smectites: A molecular investigation. Dye Pigment 2018, 160, 971–982. [Google Scholar] [CrossRef]
- Kohno, Y.; Inagawa, M.; Ikoma, S.; Shibata, M.; Matsushima, R.; Fukuhara, C.; Tomita, Y.; Maeda, Y.; Kobayashi, K. Stabilization of a hydrophobic natural dye by intercalation into organo-montmorillonite. Appl. Clay Sci. 2011, 54, 202–205. [Google Scholar] [CrossRef]
- Garcia, C.E.R.; Bolognesi, V.J.; Dias, J.D.F.G.; Miguel, O.G.; Costa, C.K. Carotenoides bixina e norbixina extraídos do urucum (Bixa orellana L.) como antioxidantes em produtos cárneos. Ciência Rural 2012, 42, 1510–1517. [Google Scholar] [CrossRef]
- Fournier, F.; Viguerie LDe Balme, S.; Janot, J.; Walter, P.; Jaber, M. Physico-chemical characterization of lake pigments based on montmorillonite and carminic acid. Appl. Clay Sci. 2016, 130, 12–17. [Google Scholar] [CrossRef]
- Trigueiro, P.; Pereira, F.A.; Guillermin, D.; Rigaud, B.; Balme, S.; Janot, J.M.; dos Santos, I.M.; Fonseca, M.G.; Walter, P.; Jaber, M. When anthraquinone dyes meet pillared montmorillonite: Stability or fading upon exposure to light? Dye Pigment 2018, 159, 384–394. [Google Scholar] [CrossRef]
- Alves, R.W. Dye extraction from annatto by adsorptive processes using commercial clays and colloidal gas aphrons. Ph.D.Thesis, Federal University of Santa Catarina, Florianópolis, Brazil, 2005. [Google Scholar]
- Madejová, J.; Gates, W.P.; Petit, S. IR Spectra of Clay Minerals. Dev. Clay Sci. 2017, 8, 107–149. [Google Scholar] [CrossRef]
- Bieseki, L.; Treichel, H.; Araujo, A.S.; Pergher, S.B.C. Porous materials obtained by acid treatment processing followed by pillaring of montmorillonite clays. Appl. Clay Sci. 2013, 85, 46–52. [Google Scholar] [CrossRef]
- do Nascimento, J.V.A. Evaluation of the Properties of Natural and Synthetic Montmorillonite Clays Pillared with Aluminum Polyhydroxications. Master’s Thesis, Federal University of Rio Grande do Norte, Natal, Brazil, 2018. [Google Scholar]
- Correa, J.H.G.; Veja, G.U.S.; dos Santos Cunha, N.; Gouveia, T.; Maciel, P.D., Jr. Um modelo simples e parametrizável para classificação de cores. Connepi 2014, 9. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).