Conductivity Transport Mechanisms of Solution-Processed Spinel Nickel Cobaltite-Based Hole Transporting Layers and Its Implementation as Charge Selective Contact in Organic Photovoltaics †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
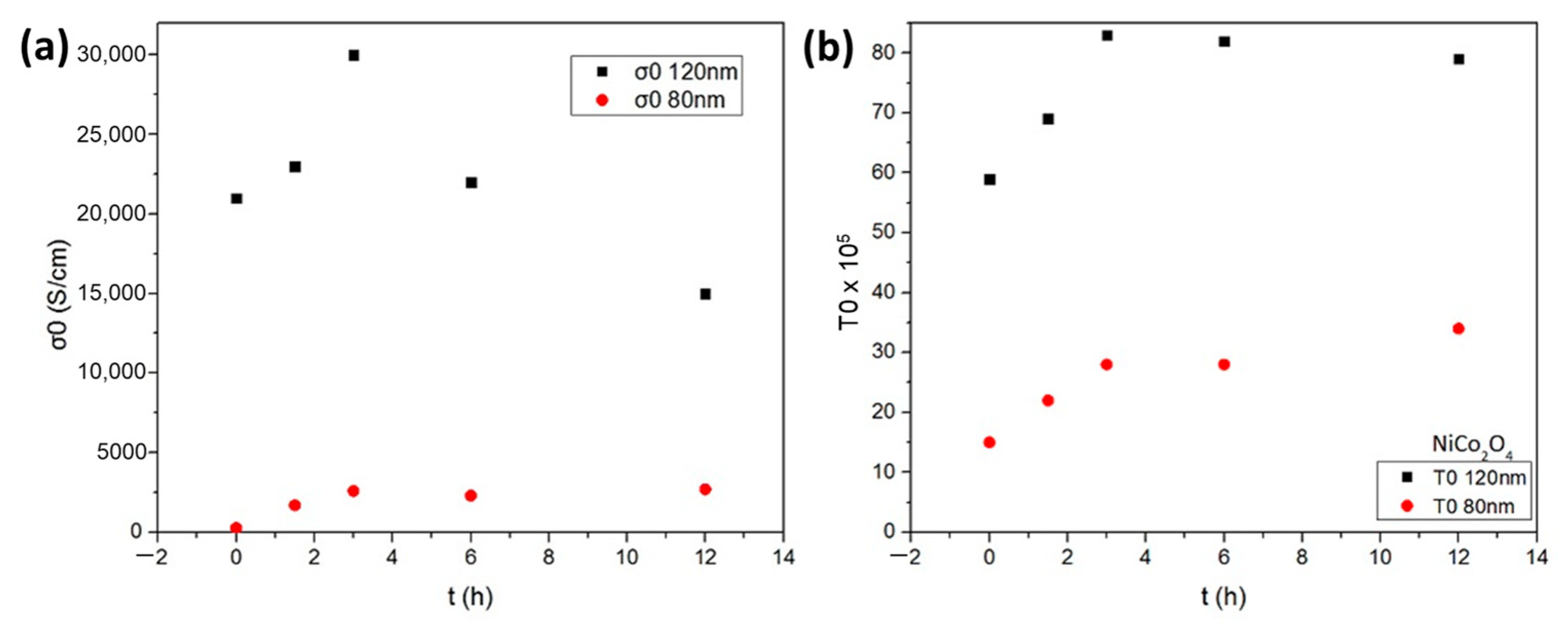
3.1. D.C. Conductivity and Hall Effect Measurements of NiCo2O4 Electronic Films
3.2. Normal Device Architecture OPVs with Neat NiCo2O4 and Cu-SCN Surface Modified NiCo2O4 HTLs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kannan, N.; Vakeesan, D. Solar energy for future world: A review. Renew. Sustain. Energy Rev. 2016, 62, 1092–1105. [Google Scholar] [CrossRef]
- ur Rehman, S.; Noman, M.; Khan, A.D.; Saboor, A.; Ahmad, M.S.; Khan, H.U. Synthesis of polyvinyl acetate/graphene nanocomposite and its application as an electrolyte in dye sensitized solar cells. Optik 2020, 202, 163591. [Google Scholar] [CrossRef]
- Lin, Y.; Shao, Y.; Dai, J.; Li, T.; Liu, Y.; Dai, X.; Xiao, X.; Deng, Y.; Gruverman, A.; Zeng, X.C.; et al. Metallic surface doping of metal halide perovskites. Nat. Commun. 2021, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, L.; Ye, F.; Zhao, T.; Tang, F.; Rajagopal, A.; Jiang, Z.; Jiang, S.; Jen, A.K.Y.; Xie, Y.; et al. Ag-Incorporated Organic-Inorganic Perovskite Films and Planar Heterojunction Solar Cells. Nano Lett. 2017, 17, 3231–3237. [Google Scholar] [CrossRef]
- Georgiou, E.; Ioakeimidis, A.; Antoniou, I.; Papadas, I.T.; Hauser, A.; Rossier, M.; Linardi, F.; Choulis, S.A. Non-Embedded Silver Nanowires/Antimony-Doped Tin Oxide/Polyethylenimine Transparent Electrode for Non-Fullerene Acceptor ITO-Free Inverted Organic Photovoltaics. ACS Appl. Electron. Mater. 2023, 5, 181–188. [Google Scholar] [CrossRef]
- Hermerschmidt, F.; Choulis, S.A.; List-Kratochvil, E.J.W. Implementing Inkjet-Printed Transparent Conductive Electrodes in Solution-Processed Organic Electronics. Adv. Mater. Technol. 2019, 4, 1800474. [Google Scholar] [CrossRef]
- Park, S.; Kim, T.; Yoon, S.; Koh, C.W.; Woo, H.Y.; Son, H.J. Progress in Materials, Solution Processes, and Long-Term Stability for Large-Area Organic Photovoltaics. Adv. Mater. 2020, 32, 2002217. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, J.; Deng, W.; Luo, M.; Xie, Y.; Liang, Q.; Zou, Y.; He, Z.; Wu, H.; Cao, Y. High-efficiency organic solar cells with low non-radiative recombination loss and low energetic disorder. Nat. Photonics 2020, 14, 300–305. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, M.; Xu, J.; Li, C.; Yan, J.; Zhou, G.; Zhong, W.; Hao, T.; Song, J.; Xue, X.; et al. Single-junction organic solar cells with over 19% efficiency enabled by a refined double-fibril network morphology. Nat. Mater. 2022, 21, 656–663. [Google Scholar] [CrossRef]
- Bao, S.; Yang, H.; Fan, H.; Zhang, J.; Wei, Z.; Cui, C.; Li, Y. Volatilizable Solid Additive-Assisted Treatment Enables Organic Solar Cells with Efficiency over 18.8% and Fill Factor Exceeding 80%. Adv. Mater. 2021, 33, 2105301. [Google Scholar] [CrossRef]
- Po, R.; Carbonera, C.; Bernardi, A.; Camaioni, N. The role of buffer layers in polymer solar cells. Energy Environ. Sci. 2011, 4, 285–310. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Ding, K.; Sheriff, H.K.M.; Ye, L.; Liu, H.; Li, C.-Z.; Ade, H.; Forrest, S.R. Non-fullerene acceptor organic photovoltaics with intrinsic operational lifetimes over 30 years. Nat. Commun. 2021, 12, 5419. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, E.; Papadas, I.T.; Antoniou, I.; Oszajca, M.F.; Hartmeier, B.; Rossier, M.; Luechinger, N.A.; Choulis, S.A. Antimony doped tin oxide/polyethylenimine electron selective contact for reliable and light soaking-free high performance inverted organic solar cells. APL Mater. 2019, 7, 91103. [Google Scholar] [CrossRef]
- Ioakeimidis, A.; Hauser, A.; Rossier, M.; Linardi, F.; Choulis, S.A. High-performance non-fullerene acceptor inverted organic photovoltaics incorporating solution processed doped metal oxide hole selective contact. Appl. Phys. Lett. 2022, 120, 233301. [Google Scholar] [CrossRef]
- Sorrentino, R.; Kozma, E.; Luzzati, S.; Po, R. Interlayers for non-fullerene based polymer solar cells: Distinctive features and challenges. Energy Environ. Sci. 2021, 14, 180–223. [Google Scholar] [CrossRef]
- Shrotriya, V.; Li, G.; Yao, Y.; Chu, C.W.; Yang, Y. Transition metal oxides as the buffer layer for polymer photovoltaic cells. Appl. Phys. Lett. 2006, 88, 73508. [Google Scholar] [CrossRef]
- Irwin, M.D.; Buchholz, D.B.; Hains, A.W.; Chang, R.P.H.; Marks, T.J. p-Type semiconducting nickel oxide as an efficiency-enhancing anode interfacial layer in polymer bulk-heterojunction solar cells. Proc. Natl. Acad. Sci. USA 2008, 105, 2783–2787. [Google Scholar] [CrossRef]
- Han, S.; Shin, W.S.; Seo, M.; Gupta, D.; Moon, S.J.; Yoo, S. Improving performance of organic solar cells using amorphous tungsten oxides as an interfacial buffer layer on transparent anodes. Org. Electron. 2009, 10, 791–797. [Google Scholar] [CrossRef]
- Wang, K.; Ren, H.; Yi, C.; Liu, C.; Wang, H.; Huang, L.; Zhang, H.; Karim, A.; Gong, X. Solution-processed Fe3O4 magnetic nanoparticle thin film aligned by an external magnetostatic field as a hole extraction layer for polymer solar cells. ACS Appl. Mater. Interfaces 2013, 5, 10325–10330. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, W.; Fu, W.; Zhang, Z.; Yang, W.; Wang, S.; Li, H.; Xu, M.; Chen, H. An aqueous solution-processed CuOX film as an anode buffer layer for efficient and stable organic solar cells. J. Mater. Chem. A 2016, 4, 5130–5136. [Google Scholar] [CrossRef]
- Gama, L.; Ribeiro, M.A.; Barros, B.S.; Kiminami, R.H.A.; Weber, I.T.; Costa, A.C.F.M. Synthesis and characterization of the NiAl2O4, CoAl2O4 and ZnAl2O4 spinels by the polymeric precursors method. J. Alloys Compd. 2009, 483, 453–455. [Google Scholar] [CrossRef]
- Xu, H.; Xu, H.; Yuan, F.; Zhou, D.; Liao, X.; Chen, L.; Chen, Y.; Chen, Y. Hole transport layers for organic solar cells: Recent progress and prospects. J. Mater. Chem. A 2020, 8, 11478–11492. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, Y.; Ji, X. NiCo2O4-based materials for electrochemical supercapacitors. J. Mater. Chem. A 2014, 2, 14759–14772. [Google Scholar] [CrossRef]
- Papadas, I.T.; Ioakeimidis, A.; Armatas, G.S.; Choulis, S.A. Low temperature combustion synthesis of a spinel NiCo2O4 hole transport layer for perovskite photovoltaics. Adv. Sci. 2018, 5, 1701029. [Google Scholar] [CrossRef] [PubMed]
- Dalas, E.; Mougoyannis, P.; Sakkopoulos, S. Εffect of ΖnO concentration on the structure and charge transport in conductive polypyrrole/ΖnO x% w/w composites with x = 10, 20, 30 and 40. Rom. J. Phys. 2013, 58, 354–364. [Google Scholar]
- Dileep, K.; Loukya, B.; Silwal, P.; Gupta, A.; Datta, R. Probing optical band gaps at nanoscale from tetrahedral cation vacancy defects and variation of cation ordering in NiCo2O4 epitaxial thin films. J. Phys. D Appl. Phys. 2014, 47, 405001. [Google Scholar] [CrossRef]
- Hu, L.; Wu, L.; Liao, M.; Fang, X. High-performance NiCo2O4 nanofilm photodetectors fabricated by an interfacial self-assembly strategy. Adv. Mater. 2011, 23, 1988–1992. [Google Scholar] [CrossRef]
- Hu, L.; Wu, L.; Liao, M.; Hu, X.; Fang, X. Electrical transport properties of large, individual NiCo2O4 nanoplates. Adv. Funct. Mater. 2012, 22, 998–1004. [Google Scholar] [CrossRef]
- Vitoratos, E.; Sakkopoulos, S.; Dalas, E.; Paliatsas, N.; Karageorgopoulos, D.; Petraki, F.; Kennou, S.; Choulis, S.A. Thermal degradation mechanisms of PEDOT:PSS. Org. Electron. 2009, 10, 61–66. [Google Scholar] [CrossRef]
- Vitoratos, E.; Sakkopoulos, S.; Dalas, E.; Emmanouil, K. Conductivity Degradation Study of polypyrrole and polypyrrole/5% w/w TiO2 nanocomposite under Heat Treatment in Helium and Atmospheric Air. Int. J. Eng. Appl. Sci. 2016, 3, 13–16. [Google Scholar]

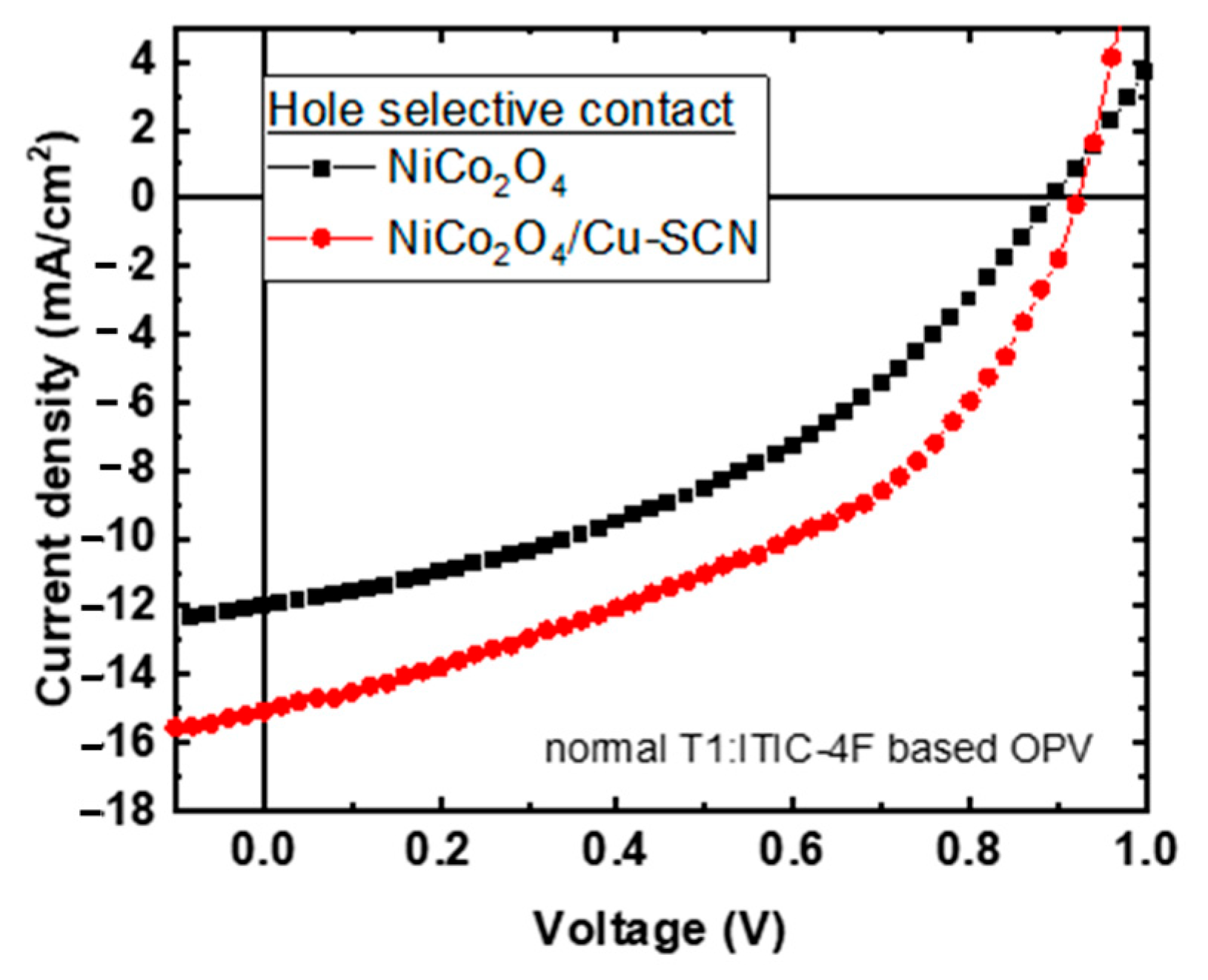
| HTL | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| NiCo2O4 | 0.89 | −12.00 | 40.75 | 4.38 |
| NiCo2O4/Cu-SCN | 0.92 | −15.06 | 44.76 | 6.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioakeimidis, A.; Kottaras, A.; Karageorgopoulos, D.; Christia, E.; Sakkopoulos, S.; Vitoratos, E.; Choulis, S.A.; Papadas, I.T. Conductivity Transport Mechanisms of Solution-Processed Spinel Nickel Cobaltite-Based Hole Transporting Layers and Its Implementation as Charge Selective Contact in Organic Photovoltaics. Environ. Sci. Proc. 2023, 26, 63. https://doi.org/10.3390/environsciproc2023026063
Ioakeimidis A, Kottaras A, Karageorgopoulos D, Christia E, Sakkopoulos S, Vitoratos E, Choulis SA, Papadas IT. Conductivity Transport Mechanisms of Solution-Processed Spinel Nickel Cobaltite-Based Hole Transporting Layers and Its Implementation as Charge Selective Contact in Organic Photovoltaics. Environmental Sciences Proceedings. 2023; 26(1):63. https://doi.org/10.3390/environsciproc2023026063
Chicago/Turabian StyleIoakeimidis, Apostolos, Aristeidis Kottaras, Dimitrios Karageorgopoulos, Efstathia Christia, Sotirios Sakkopoulos, Evangelos Vitoratos, Stelios A. Choulis, and Ioannis T. Papadas. 2023. "Conductivity Transport Mechanisms of Solution-Processed Spinel Nickel Cobaltite-Based Hole Transporting Layers and Its Implementation as Charge Selective Contact in Organic Photovoltaics" Environmental Sciences Proceedings 26, no. 1: 63. https://doi.org/10.3390/environsciproc2023026063
APA StyleIoakeimidis, A., Kottaras, A., Karageorgopoulos, D., Christia, E., Sakkopoulos, S., Vitoratos, E., Choulis, S. A., & Papadas, I. T. (2023). Conductivity Transport Mechanisms of Solution-Processed Spinel Nickel Cobaltite-Based Hole Transporting Layers and Its Implementation as Charge Selective Contact in Organic Photovoltaics. Environmental Sciences Proceedings, 26(1), 63. https://doi.org/10.3390/environsciproc2023026063






