Abstract
Supercritical fluid extraction (SCFE) is an ever-expanding approach that can be applied both for extracting components of high commercial value from natural matrices or removal of undesirable or toxic components from many types of matrices. When it comes to the removal of contaminants from natural matrices, a great deal of research has been limited to the solid residues, where both supercritical water (SCW) and supercritical carbon dioxide (SCCO2) are valid choices for extracting medium. This review, instead, focuses on the treatment of liquid matrices by SCCO2.
1. Introduction
Remediation of contaminated environmental matrices is an important task that aims to recover the areas where old highly polluting industries used to be located, the sites of urban waste discharge, or for continuous treatment of wastewater in the industrial process.
The use of supercritical fluids as solvents for the extraction of contaminants has been studied recently because it offers certain advantages over other techniques. Supercritical fluids have physicochemical properties especially attractive for the extraction process, such as high diffusivity, and compressibility which results in tunable solvent power and solvent polarity. There are two main solvents that are typically used, water and carbon dioxide. Both solvents are considered as being “green” since they are non-toxic to humans or the environment, safe to handle, and of natural origin. They reach the supercritical state when both temperature and pressure exceed the values of their corresponding critical points, for water Tc = 374 °C, Pc = 217.7 atm and for CO2 Tc = 31 °C, Pc = 72.8 atm. As can be seen from these values, the condition necessary for SCW are very harsh, so its use is limited for technical and economic reasons.
The variety of organic solutes that can be extracted from aqueous and solid waste (wastewater, contaminated soils, sludges, etc.) by SCFs as well as the availability of inexpensive and nontoxic SCF solvents such as CO2 has positioned this process as a viable method for removing toxic organic compounds.
2. Treatment of Aqueous Waste by Supercritical Fluids
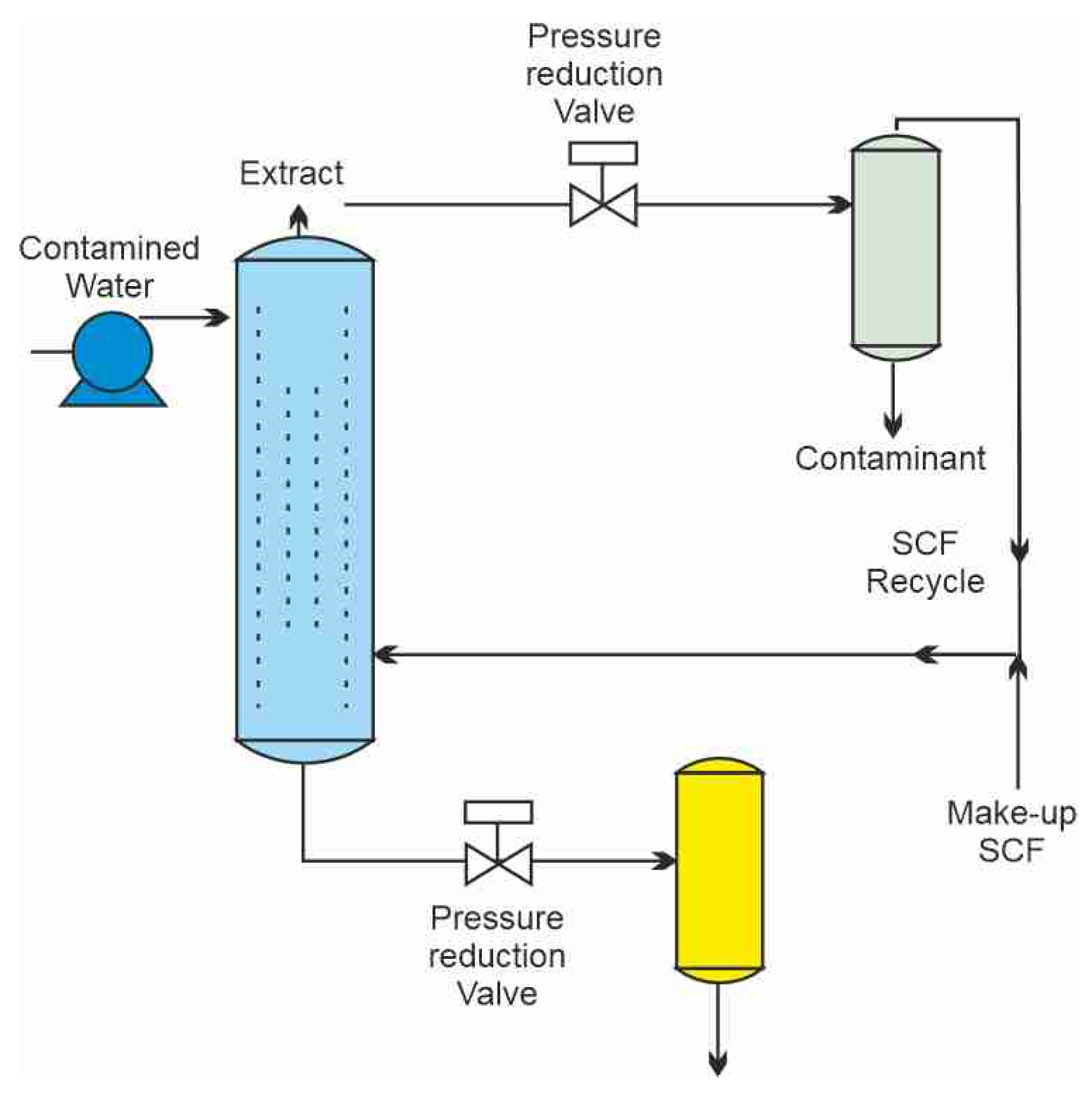
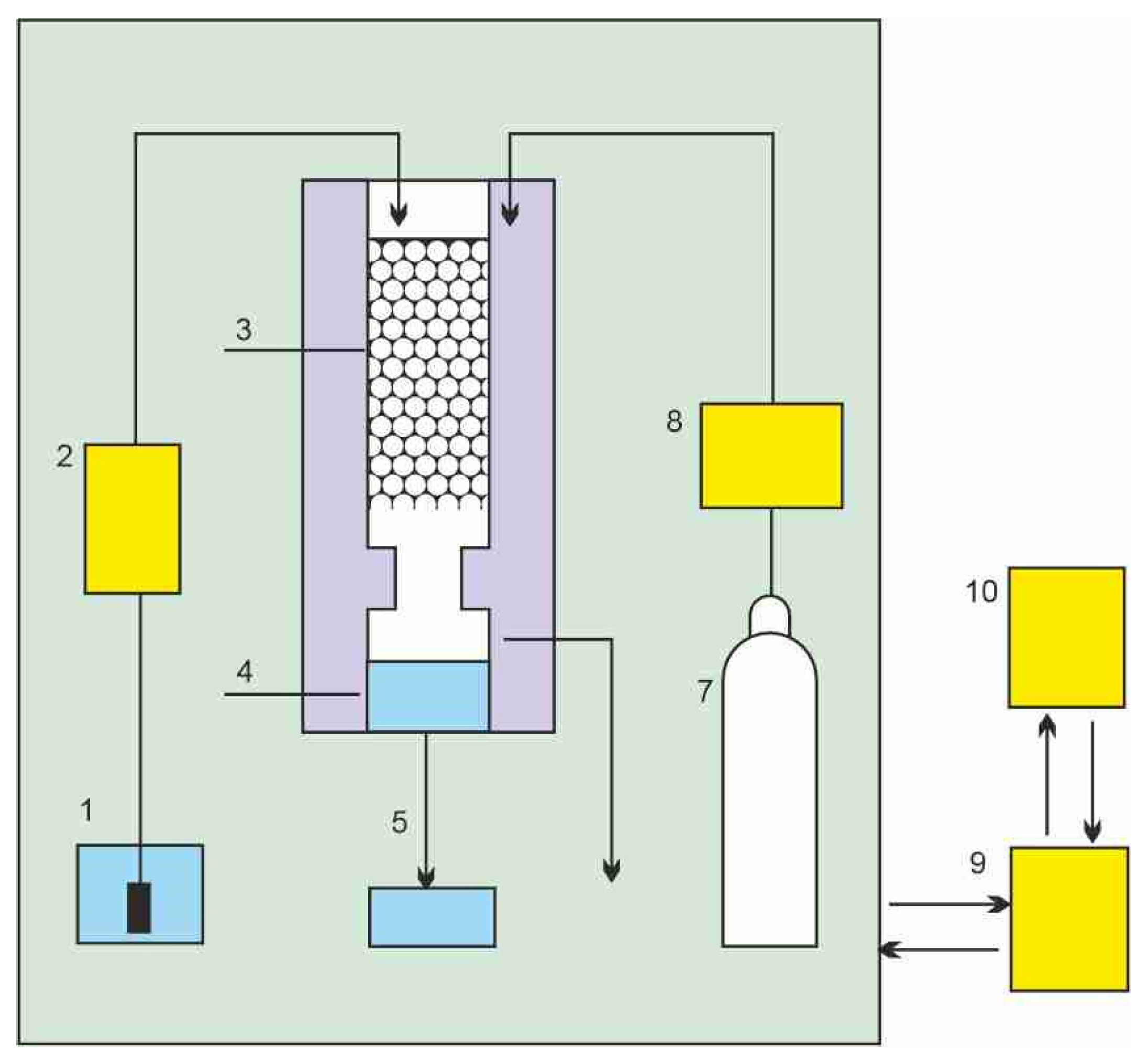
The schematics of a treatment process of contaminated wastewater by SCCO2 are shown in Figure 1 [1]. Carbon dioxide is pressurized and heated above its critical point to bring it to the supercritical condition. The flow of SCCO2 is introduced near the bottom of a separation column, while contaminated water flow enters at the top as a countercurrent gravity-driven flow, since water density is higher than that of SCCO2. Carbon dioxide with extracted organic contaminants leaves the column at the top and is expanded across the pressure reduction valve, where carbon dioxide turns into a gas and the extracted contaminants precipitate in a separator vessel. Carbon dioxide can be either vented into the atmosphere or recycled. Decontaminated water is collected at the bottom of the separation column and also depressurized, at which point the dissolved CO2 can also be collected and reused. A different approach that places the two flows co-current with each other has been proposed by Karasek et al. [2] as shown in Figure 2.
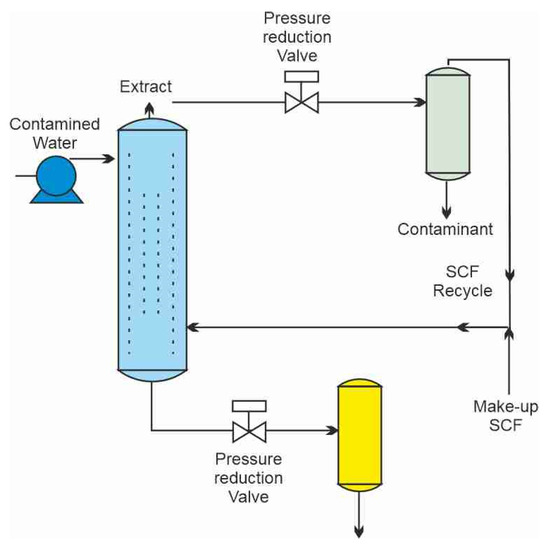
Figure 1.
A supercritical extraction process for the extraction of organics from water [1].
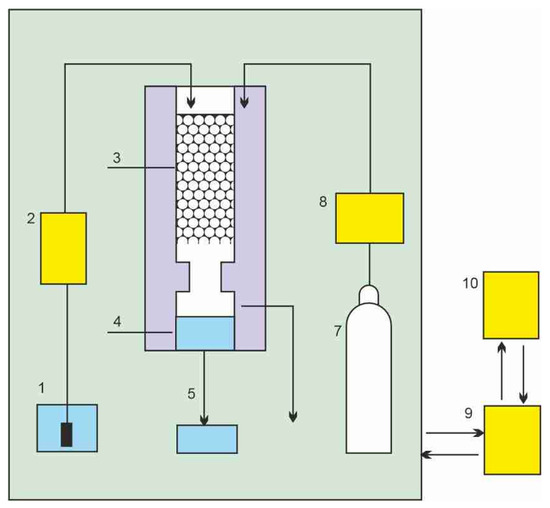
Figure 2.
Schematic diagram of the extraction cell and the phase separator: 1, aqueous-phase reservoir; 2, aqueous-phase pump; 3, extraction column; 4, phase separator; 5, aqueous-phase restrictor; 6, CO2-rich phase restrictor; 7, CO2 cylinder; 8, liquid CO2 pump; 9, control unit; 10, PC for input and display of operating parameters [2].
In order to improve the separation of the contaminants between the two phases column height, countercurrent flow regime and physical parameters (pressure, temperature, or temperature gradient) must be optimized. Column packing can be used to ensure better mixing and reaching equilibrium conditions faster.
A key parameter for any extraction is the distribution coefficient, which describes the equilibrium concentration ratio for any given substance between the two solvent phases. The distribution coefficient depends mostly on the polarity of the contaminant. Since SCCO2 is a nonpolar solvent, as opposed to water, it is very efficient in the extraction of nonpolar substances such as benzene, and less efficient for relatively polar phenols Table 1 [1,3].

Table 1.
Distribution coefficients of organic compounds for the water-SCCO2 system.
A way to make SCCO2 a better solvent for more polar compounds is an addition of small amounts of other solvents, called entrainers. This approach is commonly used in SCFE of solid matrices, methanol and ethanol being the most reported entrainers. There is very little data in that regard, one study shows that methanol is not effective as an entrainer, while benzylic compounds increase the distribution coefficients of other benzylic compounds [4,5,6]. That fact suggests, that an entrainer should not be water soluble to be effective.
Besides organic compounds, SCFE can remove ions of heavy metal from both solid and liquid matrices. Nonpolar organic ligands that make complexes with these ions are used to make them soluble in SCCO2 [7].
3. Conclusions
SCFE was shown to have great potential as a green treatment approach for contaminated environmental matrices. The treatment of wastewater has been studied much less than the treatment of solid matrices, such as sediments, sands, etc. SCCO2 is the best solvent for the removal of highly nonpolar contaminants, and with the use of entrainers can be applied for the removal of slightly polar substances and heavy metals. However, there is a need for further studies and process optimization of this application.
Author Contributions
Writing—original draft preparation, P.K.; writing—review and editing, C.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akgerman, A. Supercritical fluid extraction of contaminants from environmental matrices. Waste Manag. 1993, 13, 403–415. [Google Scholar] [CrossRef]
- Karasek, P.; Pol, J.; Planeta, J.; Roth, M.; Vejrosta, J.; Wicar, S. Partition Coefficients of Environmentally Important Phenols in a Supercritical Carbon Dioxide-Water System from Cocurrent Extraction without Analysis of the Compressible Phase. Anal. Chem. 2002, 74, 4294–4299. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ghonasgi, D.; Dooley, K.M.; Knopf, F.C. Supercritical Carbon Dioxide Extraction of a Phenolic Mixture from an Aqueous Waste Stream. J. Supercrit. Fluids. 1991, 4, 181–185. [Google Scholar] [CrossRef]
- Roop, R.K.; Akgerman, A. Entrainer Effect for Supercritical Extraction of Phenol from Water. Ind. Eng. Chem. Res. 1989, 28, 1542–1546. [Google Scholar] [CrossRef]
- Yen, S.-D.; Akgerman, A. Supercritical Extraction of Organic Mixtures from Aqueous Solutions. AIChE J. 1990, 36, 1743–1747. [Google Scholar]
- Roop, R.K.; Akgerman, A. Distribution of a Complex Phenolic Mixture between Water and Supercritical Carbon Dioxide. J. Chem. Eng. Data. 1990, 35, 257–260. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Ansari, K.; Ghaziaskar, H.S. Supercritical extraction of toxic heavy metals from aqueous waste via Cyanex 301 as chelating agent. J. Supercrit. Fluids. 2012, 72, 288–297. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).