DNA Takes Over on the Control of the Morphology of the Composite Self-Organized Structures of Barium and Calcium Silica–Carbonate Biomorphs, Implications for Prebiotic Chemistry on Earth
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of DNA
2.1.1. E. coli
2.1.2. C. albicans
2.2. Visualization of DNA in Agarose Gel
2.3. Spectrophotometer Analysis
2.4. Biomorph Synthesis
2.4.1. Control Biomorphs
2.4.2. Biomorphs with Kaolinite and Genomic DNA
2.5. Characterization of the Biomorphs
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. Raman Spectroscopy
2.5.3. Fourier Transform Infrared Spectroscopy (FTIR)
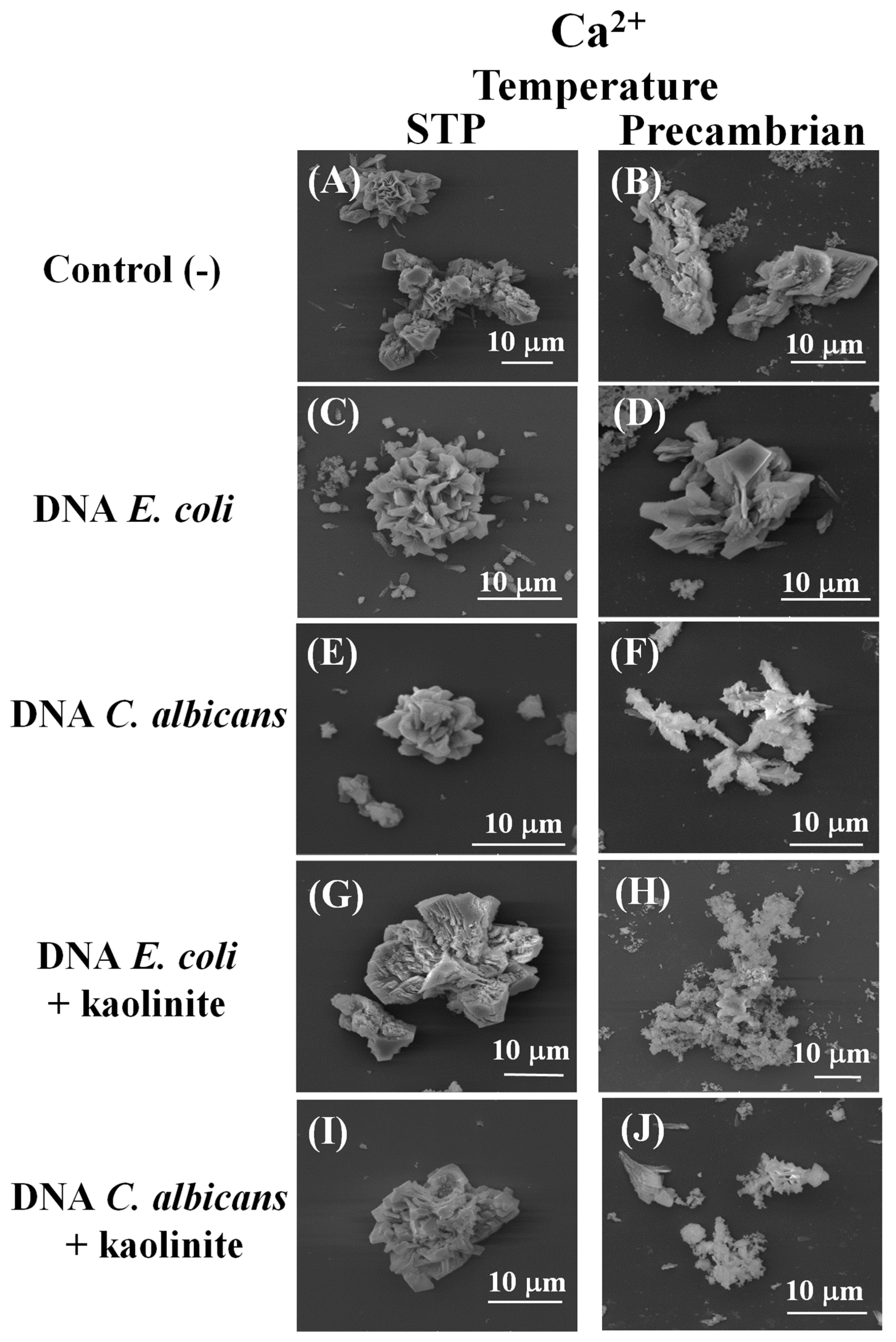
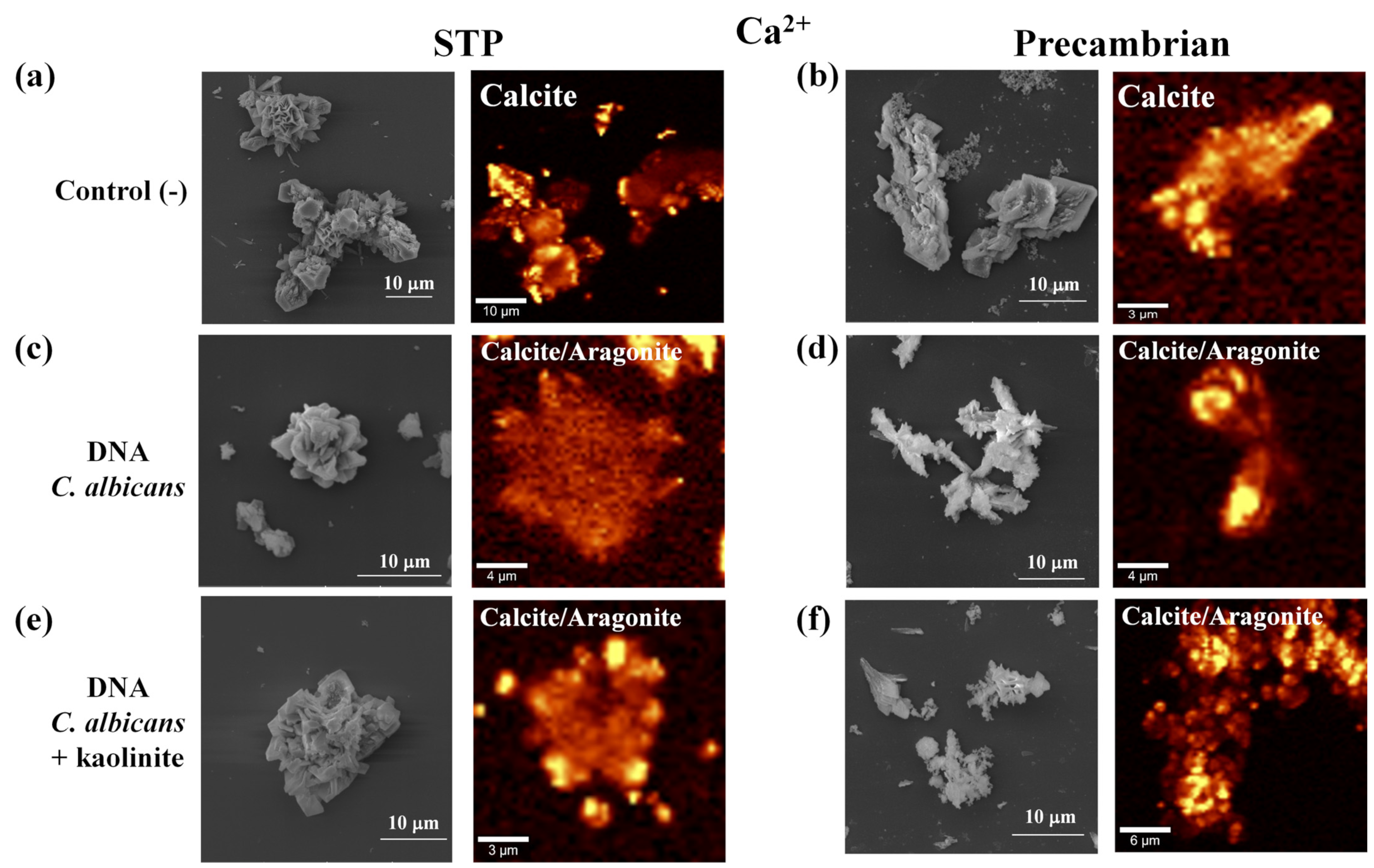
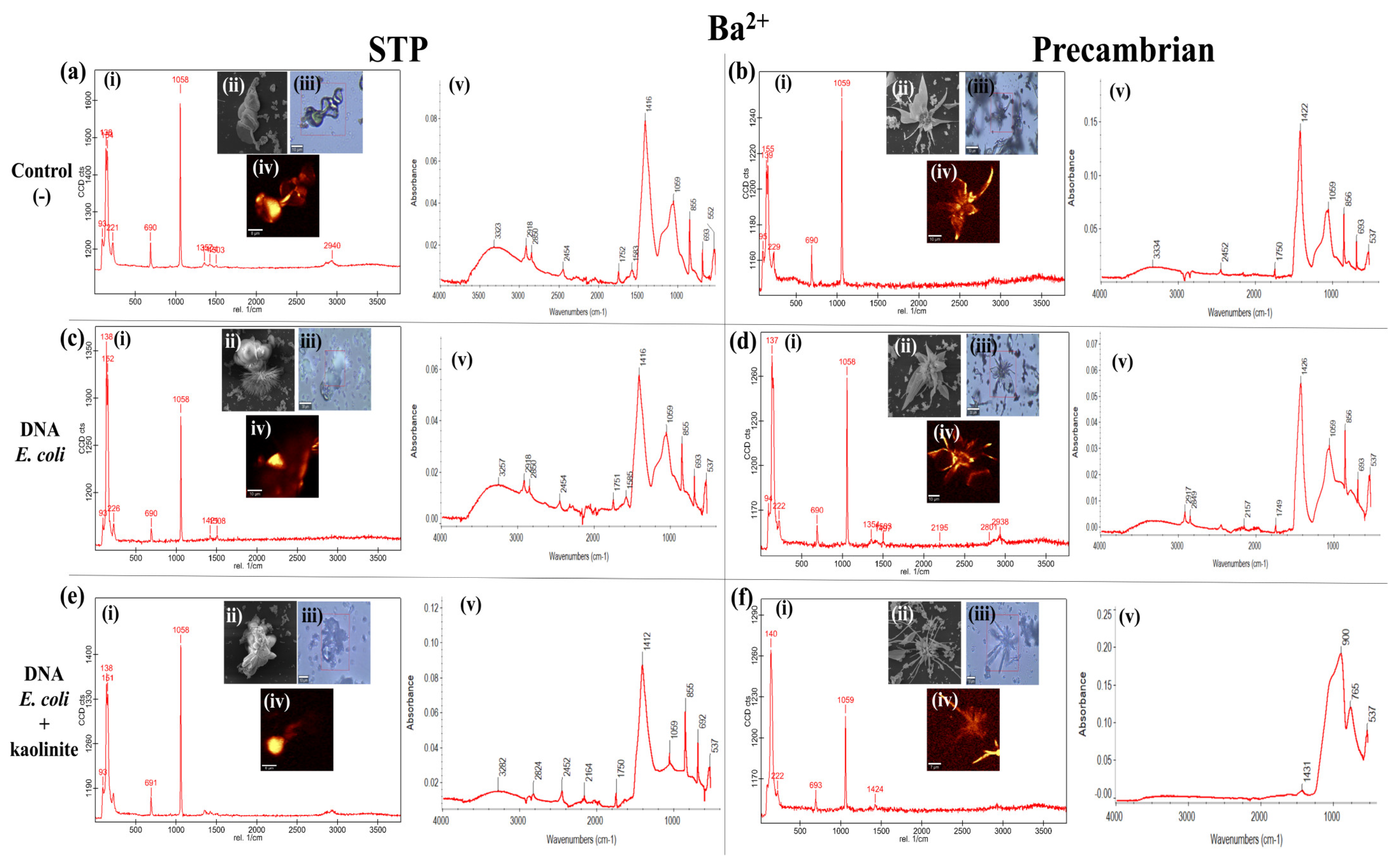
3. Results and Discussion
3.1. The Difference between the Prokaryotic and the Eukaryotic DNA Favors the Morphology and Crystalline Phase of the Silica–Carbonates of Calcium
3.2. Environmental Factors That Participate Directly in the Morphology of the BaCO3 Biomorphs but Not in the Crystalline Phase
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jenkins, R.J.F.; Ford, C.H.; Gehling, J.G. The Ediacara Member of the Rawnsley Quartzite: The context of the Ediacara assemblage (late Precambrian, Flinders Ranges). J. Geol. Soc. Aust. 1983, 30, 101–119. [Google Scholar] [CrossRef]
- Wächtershäuser, G. From volcanic origins of chemoautotrophic life to Bacteria, Archaea and Eukarya. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1787–1808. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar-Cruz, M. The equation of life in the Universe: Biomorphs as reminiscence of the first forms of life. Prog. Cryst. Growth Charact. Mater. 2024, 70, 100624. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M. The equation of the origin in the Universe (Part II): The combination of chemical elements does not determine the emergence of life on Earth. Prog. Cryst. Growth Charact. Mater. 2024, 70, 100625. [Google Scholar] [CrossRef]
- Ulrich, B. Soil Acidity and its Relations to Acid Deposition. In Effects of Accumulation of Air Pollutants in Forest Ecosystems; Ulrich, B., Pankrath, J., Eds.; Springer: Dordrecht, The Netherlands, 1983; pp. 127–146. [Google Scholar]
- Dixon, J.B. Kaolin and Serpentine group minerals. In Minerals in Soil Environments, 2nd ed.; Dixon, J.B., Weed, S.B., Eds.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 1989; Volume 1, pp. 467–525. [Google Scholar]
- Moore, D.M.; Reynolds, R.J. X-ray Diffraction and the Identification and Analysis of Clay Minerals. In State Geological Survey; Oxford University Press: Oxford, UK, 1989; p. 332. [Google Scholar]
- Lázaro, B.B. Halloysite and kaolinite: Two clay minerals with geological and technological importance. Rev. Acad. Cienc. Exactas Fis. Quim. Nat. Zaragoza 2015, 70, 7–38. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to Rock-Forming Minerals; Longman Group Ltd.: London, UK, 1975; p. 528. [Google Scholar]
- Swindale, L.D. The crystallography of minerals of the kaolin group. In Soil Components; Inorganic Components; Springer: Berlin/Heidelberg, Germany, 1975; Volume 2, pp. 121–154. [Google Scholar]
- Ehlmann, B.L.; Swayze, G.A.; Milliken, R.E.; Mustard, J.F.; Clark, R.N.; Murchie, S.L.; Breit, G.N.; Wray, J.J.; Gondet, B.; Poulet, F.; et al. Discovery of alunite in Cross Crater, Terra Sirenum, Mars: Evidence for acidic, sulfurous waters. Am. Min. 2016, 101, 1527–1542. [Google Scholar] [CrossRef]
- Pineau, M.; Mathian, M.; Baron, F.; Rondeau, B.; Deit, L.L.; Allard, T.; Mangold, N. Estimating kaolinite crystallinity using near-infrared spectroscopy: Implications for its geology on Earth and Mars. Am. Min. 2022, 107, 1453–1469. [Google Scholar] [CrossRef]
- Murchie, S.; Arvidson, R.; Bedini, P.; Beisser, K.; Bibring, J.-P.; Bishop, J.; Boldt, J.; Cavender, P.; Choo, T.; Clancy, R.T.; et al. Compact Reconnaissance Imaging Spectrometer for Mars (CRISM) on Mars Reconnaissance Orbiter (MRO). J. Geophys. Res. 2007, 112, 1–57. [Google Scholar] [CrossRef]
- Mustard, J.F.; Murchie, S.L.; Pelkey, S.M.; Ehlmann, B.L.; Milliken, R.E.; Grant, J.A.; Bibring, J.-P.; Poulet, F.; Bishop, J.; Dobrea, E.N.; et al. Hydrated silicate minerals on Mars observed by the Mars Reconnaissance Orbiter CRISM instrument. Nature 2008, 454, 305–309. [Google Scholar] [CrossRef]
- Carter, J.; Quantin, C.; Thollot, P.; Loizeau, D.; Ody, A.; Lozach, L. Oxia Planum, a clay-laden landing site proposed for the Exomars Rover Mission: Aqueous mineralogy and alteration scenarios. In Proceedings of the 47th Annual Lunar and Planetary Science Conference, The Woodlands, TX, USA, 21–25 March 2016; p. 2064. [Google Scholar]
- Ehlmann, B.L.; Edwards, C.S. Mineralogy of the Martian surface. Annu. Rev. Earth Planet. Sci. 2014, 42, 291–315. [Google Scholar] [CrossRef]
- Carrascoza Mayén, J.F.; Rydzewski, J.; Szostak, N.; Blazewicz, J.; Nowak, W. Prebiotic Soup Components Trapped in Montmorillonite Nanoclay Form New Molecules: Car-Parrinello Ab Initio Simulations. Life 2019, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Nayak, A. Kamaluddin Catalytic Role of Manganese Oxides in Prebiotic Nucleobases Synthesis from Formamide. Orig. Life Evol. Biosph. 2016, 46, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ritson, D.J.; Battilocchio, C.; Ley, S.V.; Sutherland, J.D. Mimicking the Surface and Prebiotic Chemistry of Early Earth Using Flow Chemistry. Nat. Commun. 2018, 9, 1821. [Google Scholar] [CrossRef] [PubMed]
- Rouillard, J.; Van Kranendonk, M.J.; Lalonde, S.; Gong, J.; van Zuilen, M.A. Correlating trace element compositions, petrology, and Raman spectroscopy data in the ∼3.46 Ga Apex chert, Pilbara Craton, Australia. Precambrian Res. 2021, 366, 106415. [Google Scholar] [CrossRef]
- Schopf, J.W.; Packer, B.M. Early Archean (3.3-Billion to 3.5-Billion-Year-Old) Microfossils from Warrawoona Group, Australia. Science 1987, 237, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Schopf, J.W. Microfossils of the Early Archean Apex Chert: New Evidence of the Antiquity of Life. Science 1993, 260, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Schopf, W.J.; Kudryavtsev, A.B. Biogenicity of Earth’s Earliest Fossils: A Resolution of the Controversy. Gondwana Res. 2012, 22, 761–771. [Google Scholar] [CrossRef]
- Brasier, M.D.; Green, O.R.; Jephcoat, A.P.; Kleppe, A.K.; Van Kranendonk, M.J.; Lindsay, J.F.; Steele, A.; Grassineau, N.V. Questioning the Evidence for Earth’s Oldest Fossils. Nature 2002, 416, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Brasier, M.D.; McLoughlin, N.; Green, O.; Wacey, D. A Fresh Look at the Fossil Evidence for Early Archaean Cellular Life. Philos. Trans. R. Soc. Lond. Ser. B 2006, 361, 887–902. [Google Scholar] [CrossRef]
- Brasier, M.D.; Antcliffe, J.; Saunders, M.; Wacey, D. Changing the picture of Earth’s earliest fossils (3.5–1.9 Ga) with new approaches and new discoveries. Proc. Natl. Acad. Sci. USA 2015, 112, 4859–4864. [Google Scholar] [CrossRef]
- Pinti, D.L.; Mineau, R.; Clement, V. Hydrothermal Alteration and Microfossil Artefacts of the 3465-Million-Year-Old Apex Chert. Nat. Geosci. 2009, 2, 640–643. [Google Scholar] [CrossRef]
- Marshall, C.P.; Emry, J.R.; Olcott Marshall, A. Haematite Pseudomicrofossils Present in the 3.5-Billion-Year-Old Apex Chert. Nat. Geosci. 2011, 4, 240–243. [Google Scholar] [CrossRef]
- Sforna, M.C.; van Zuilen, M.A.; Philippot, P. Structural Characterization by Raman Hyperspectral Mapping of Organic Carbon in the 3.46 Billion-Year-Old Apex Chert, Western Australia. Geochim. Cosmochim. Acta 2014, 124, 18–33. [Google Scholar] [CrossRef]
- Garcia-Ruiz, J.M.; Hyde, S.T.; Carnerup, A.M.; Christy, A.G.; Van Kranendonk, V.M.J.; Welham, N.J. Self-assembled silicacarbonate structures and detection of ancient microfossils. Science 2003, 302, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Opel, J.; Wimmer, F.P.; Kellermeier, M.; Colfen, H. Functionalisation of Silica-Carbonate Biomorphs. Nanoscale Horiz. 2016, 1, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Voinescu, A.E.; Kellermeier, M.; Carnerup, A.M.; Larsson, A.K.; Touraud, D.; Hyde, S.T.; Kunz, W. Co-Precipitation of Silica and Alkaline-Earth Carbonates Using TEOS as Silica Source. J. Cryst. Growth 2007, 306, 152–158. [Google Scholar] [CrossRef]
- Voinescu, A.E.; Kellermeier, M.; Bartel, B.; Carnerup, A.M.; Larsson, A.K.; Touraud, D.; Kunz, W.; Kienle, L.; Pfitzner, A.; Hyde, S.T. Inorganic Self-Organized silica aragonite biomorphic composites. Cryst. Growth Des. 2008, 8, 1515–1521. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M. New insights on the origin of life: The role of silico-carbonates of Ba (II) to preserve DNA against highly intense UV radiation. ACS Omega 2023, 8, 29585–29594. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar-Cruz, M.; Islas, S.R.; Ramírez-Ramírez, N.; Pedraza-Reyes, M.; Moreno, A. Protection of the DNA from selected species of five kingdoms in Nature by Ba(II), Sr(II) and Ca(II) silica-carbonates: Implications about biogenicity and evolving from prebiotic chemistry to biological chemistry. ACS Omega 2022, 7, 37410–37426. [Google Scholar] [CrossRef]
- Cuéllar-Arcos, J.C.; Islas, S.R.; Cuéllar-Cruz, M. Biomorphs possess mineral crystalline plasticity by adapting their morphology and chemical composition in the presence of organic biomolecules and inorganic ions. ACS Earth Space Chem. 2023, 7, 2535–2547. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M.; Islas, S.R.; González, G.; Moreno, A. Influence of nucleic acids on the synthesis of crystalline Ca (II), Ba (II), and Sr (II) silica-carbonate biomorphs: Implications for the chemical origin of life on primitive Earth. Cryst. Growth Des. 2019, 19, 4667–4682. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M.; Moreno, A. The role of calcium and strontium as the most dominant elements during combinations of different alkaline Earth metals in the synthesis of crystalline silica-carbonate biomorphs. Crystals 2019, 9, 381. [Google Scholar] [CrossRef]
- Islas, S.R.; Cuéllar-Cruz, M. Silica-carbonate of Ba(II) and Fe2+/Fe3+ Complex as Study Models to Understand Prebiotic Chemistry. ACS Omega 2021, 6, 35629–35640. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar-Cruz, M. Influence of Abiotic Factors in the Chemical Origin of Life: Biomorphs as a Study Model. ACS Omega 2021, 6, 8754–8763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Morales, J.; Garcia-Ruiz, J.M. Growth Behaviour of Silica/Carbonate Nanocrystalline Composites of Calcite and Aragonite. J. Mater. Chem. B 2017, 5, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Bittarello, E.; Aquilano, D. Self-assembled nanocrystals of barium carbonate in biomineral-like structures. Eur. J. Mineral. 2007, 19, 345–351. [Google Scholar] [CrossRef]
- Bittarello, E.; Roberto Massaro, F.R.; Aquilano, D. The epitaxial role of silica groups in promoting the formation of silica/carbonate biomorphs: A first hypothesis. J. Cryst. Growth 2010, 312, 402–412. [Google Scholar] [CrossRef]
- Bittarello, E.; Massaro, F.R.; Rubbo, M.; Costa, E.; Aquilano, D. Witherite (BaCO3)/α-Quartz epitaxial nucleation and growth: Experimental findings and theorical implications on biomineralization. Cryst. Growth Des. 2009, 9, 971–977. [Google Scholar] [CrossRef]
- Bruno, M.; Rubbo, M.; Pastero, L.; Massaro, F.R.F.; Nestola, D.; Aquilano, D. Computational Approach to the Study of Epitaxy: Natural Occurrence in Diamond/Forsterite and Aragonite/Zabuyelite. Cryst. Growth Des. 2015, 15, 2979–2987. [Google Scholar] [CrossRef]
- Bruno, M.; Pastero, L.; Cotellucci, A.; Aquilano, D. Epitaxy: A methodological approach to the study of an old phenomenon. Cryst Eng Comm. 2022, 24, 4165–4173. [Google Scholar] [CrossRef]
- Aquilano, D.; Bruno, M.; Ghignone, S.; Pastero, L.; Cotellucci, A. Epitaxies of Ca sulfates on calcite. II. The main {010}, {001} and {100} forms of bassanite epi-deposited on the {10.4} substrate form of calcite. J. Appl. Crystallogr. 2022, 55, 1289–1296. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M.; Ramírez-Cardona, M.; Islas, S.R.; Moreno, A. Influence of different types of clay minerals on the shape and form of silica-carbonates (Biomorphs) of Ca(II), Ba(II), AND Sr(II). ACS Earth Space Chem. 2022, 6, 3054–3065. [Google Scholar] [CrossRef]
- Zúñiga-Estrada, E.A.; Zúñiga-Estrada, M.A.; Islas, S.R.; Moreno, A.; Cuéllar-Cruz, M. Minerals participated in the formation of the first inorganic structure that favored the synthesis of protomolecules: Biomorphs as models of study. ACS Omega 2024. submitted. [Google Scholar]
- Rickwood, D.; Hames, B.D. Gel Electrophoresis of Nucleic Acids: A practical Approach; Oxford University Press: New York, NY, USA, 1990; pp. 65–66. [Google Scholar]
- López-Mora, P.A.; López Gutiérrez, A.M.; Marulanda-Ángel, M.L. Standardizing genomic DNA extraction in Tabebuia rosea (Bertol.) DC. and Cordia alliodora (Ruiz and Pav.) Okén. Temas Agrar. 2011, 16, 28–41. [Google Scholar] [CrossRef][Green Version]
- Demeke, T.; Jenkins, G.R. Influence of DNA extraction methods, PCR inhibitors and quantification methods on real-time PCR assay of biotechnology-derived traits. Anal. Bioanal. Chem. 2010, 396, 1977–1990. [Google Scholar] [CrossRef] [PubMed]
- Noorduin, W.L.; Grinthal, A.; Mahadevan, L.; Aiznberg, J. Rationally Designed Complex, Hierarchical Microarchitectures. Science 2013, 340, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar-Cruz, M. Synthesis of inorganic and organic crystals mediated by proteins in different biological organisms. A mechanism of biomineralization conserved throughout evolution in all living species. Prog. Cryst. Growth Charact. Mater. 2017, 63, 94–103. [Google Scholar] [CrossRef]
- Ruiz-Arellano, R.R.; Moreno, A. Obtainment of spherical-shaped calcite crystals induced by intramineral proteins isolated from eggshells of ostrich and emu. Cryst. Growth Des. 2014, 14, 5137–5143. [Google Scholar] [CrossRef]
- Ruiz-Arellano, R.R.; Medrano, F.J.; Moreno, A.; Romero, A. Crystal Structure of Struthiocalcin-1, an Intramineral Protein from Struthio camelus Eggshell, in two crystal forms. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 809–818. [Google Scholar] [CrossRef]
- Reyes-Grajeda, J.P.; Moreno, A.; Romero, A. Crystal structure of ovocleidin-17, a major protein of the calcified Gallus gallus eggshell: Implications in the calcite mineral growth pattern. J. Biol. Chem. 2004, 279, 40876–40881. [Google Scholar] [CrossRef]
- Cölfen, H. Biomineralization: A crystal-clear view. Nat. Mater. 2010, 9, 960–961. [Google Scholar] [CrossRef]
- Mann, K. The calcified eggshell matrix proteome of a songbird, the zebra finch (Taeniopygia guttata). Proteome Sci. 2015, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Mann, M. The proteome of the calcified layer organic matrix of turkey (Meleagris gallopavo) eggshell. Proteome Sci. 2013, 11, 40. [Google Scholar] [CrossRef]
- Jáuregui-Zúñiga, D.; Reyes-Grajeda, J.P.; Sepúlveda-Sanchez, J.D.; Whitaker, J.R.; Moreno, A. Crystallochemical characterization of calcium oxalate crystals isolated from seed coats of Phaseolus vulgaris and leaves of Vitis vinifer. J. Plant Physiol. 2003, 160, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Carteret, C.; Dandeu, A.; Moussaoui, S.; Muhr, H.; Humbert, B.; Plasari, E. Polymorphism Studied by Lattice Phonon Raman Spectroscopy and Statistical Mixture Analysis Method. Application to Calcium Carbonate Polymorphs during Batch Crystallization. Cryst. Growth Des. 2009, 9, 807–812. [Google Scholar] [CrossRef]
- Mann, S. Biomineralization: Principle and Concepts in Bioinorganic Materials Chemistry; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Lowenstam, H.A. Minerals formed by organisms. Science 1981, 211, 1126–1131. [Google Scholar] [CrossRef]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Benzerara, K.; Miot, J.; Morin, G.; Ona-Nguema, G.; Skouri-Panet, F.; Ferard, C. Significance, mechanisms and environmental implications of microbial biomineralization. Comptes Rendus Geosci. 2011, 343, 160–167. [Google Scholar] [CrossRef]
- Phillips, A.J.; Gerlach, R.; Lauchnor, E.; Mitchell, A.C.; Cunningham, A.B.; Spangler, L. Engineered applications of ureolytic biomineralization: A review. Biofouling 2013, 29, 715–733. [Google Scholar] [CrossRef] [PubMed]
- Anbu, P.; Kang, C.H.; Shin, Y.J.; So, J.S. Formations of calcium carbonate minerals by bacteria and its multiple applications. Springerplus 2016, 5, 250. [Google Scholar] [CrossRef]
- Sarikaya, M. Biomimetics: Materials fabrication through biology. Proc. Natl. Acad. Sci. USA 1999, 96, 14183–14185. [Google Scholar] [CrossRef]
- Blattner, F.R.; Plunkett, G.; Bloch, C.A.; Perna, N.T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J.D.; Rode, C.K.; Mayhew, G.F.; et al. The complete genome sequence of Escherichia coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The Genome Sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Bult, C.J.; White, O.; Olsen, G.J.; Zhou, L.; Fleischmann, R.D.; Sutton, G.G.; Blake, J.A.; FitzGerald, L.M.; Clayton, R.A.; Gocayne, J.D.; et al. Complete genome sequence of the methanogenic archaeon Methanococcus jannaschii. Science 1996, 273, 1058–1073. [Google Scholar] [CrossRef] [PubMed]
- Ahnert, S.E.; Fink, T.M.; Zinovyev, A. How much non-coding DNA do eukaryotes require? J. Theor. Biol. 2008, 252, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Bendich, A.J.; Drlica, K. Prokaryotic and eukaryotic chromosomes: What’s the difference? Bioessays 2000, 22, 481–486. [Google Scholar] [CrossRef]
- Cairns, J. The chromosome of Escherichia coli. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 1963; Volume 28, pp. 43–46. [Google Scholar]
- Mason, D.J.; Powelson, D.M. Nuclear division as observed in live bacteria by a new technique. J. Bacteriol. 1956, 71, 474–479. [Google Scholar] [CrossRef]
- Falini, G.; Albeck, S.; Weiner, S.; Addadi, L. Control of aragonite or calcite polymorphism by mollusk shell macromolecules. Science 1996, 271, 67–69. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G.; Marie, B.; Medakovic, D. Molluscan shell proteins: Primary structure, origin, and evolution. Curr. Top. Dev. Biol. 2008, 80, 209–276. [Google Scholar] [PubMed]
- Xiang, L.; Su, J.; Zheng, G.; Liang, J.; Zhang, G.; Wang, H.; Xie, L.; Zhang, R. Patterns of expression in the matrix proteins responsible for nucleation and growth of aragonite crystals in flat pearls of Pinctada fucata. PLoS ONE 2013, 8, e66564. [Google Scholar] [CrossRef]
- Montalti, M.; Zhang, G.; Genovese, D.; Morales, J.; Kellermeier, M.; Garcia-Ruiz, J.M. Local pH oscillations witness autocatalytic self-organization of biomorphic nanostructures. Nat. Commun. 2017, 8, 14427. [Google Scholar] [CrossRef]
- Melero-García, E.; Santisteban-Bailón, R.; García-Ruiz, J.M. Role of bulk pH during witherite biomorph growth in silica gels. Cryst. Growth Des. 2009, 9, 4730–4734. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; Melero-García, E.; Hyde, S.T. Morphogenesis of self-assembled nanocrystalline materials of barium carbonate and silica. Science 2009, 323, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.L.Y.; Howie, R.A.; Zussman, J. Rock forming minerals. In Non-Silicates: Sulphates, Carbonates, Phosphates and Halides, 2nd ed.; Geological Society of London: London, UK, 1998. [Google Scholar]
- Mauchline, J.; Templeton, W.L. Strontium, calcium and barium in marine organisms from the Irish Sea. ICES J. Mar. Sci. 1966, 30, 161–170. [Google Scholar] [CrossRef]
- Lin, C.C.; Liu, L.G. High-pressure Raman spectroscopic study of post-aragonite phase transition in witherite (BaCO3). Eur. J. Mineral. 1997, 9, 785–792. [Google Scholar] [CrossRef]
- Buzgar, N.; Apopei, A.I. The Raman Study of Certain Carbonates. Geol. Tomul L 2009, 2, 97–112. [Google Scholar]
- Zaoui, A.; Shahrour, I. Molecular dynamics study of high-pressure polymorphs of BaCO3. Philos. Mag. Lett. 2010, 90, 689–697. [Google Scholar] [CrossRef]
- Lin, C.C.; Liu, L. High pressure phase transformations in aragonite-type carbonates. Phys. Chem. Miner. 1997, 24, 149–157. [Google Scholar] [CrossRef]
- Holl, C.M.; Smyth, J.R.; Laustsen, H.M.S.; Jacobsen, S.D.; Downs, R.T. Compression of witherite to 8 GPa and the crystal structure of BaCO3 II. Phys. Chem. Miner. 2000, 27, 467–473. [Google Scholar]
- Jiao, J.; Liu, X.; Gao, W.; Wang, C.; Feng, H.; Zhao, X.; Chen, L. Two-step synthesis of witherite and tunin g of morphology. Mater. Res. Bull. 2010, 45, 181–185. [Google Scholar] [CrossRef]
- Shen, X.F.; Yan, X.P. Facile Shape-Controlled Synthesis of Well-Aligned Nanowire Architectures in Binary Aqueous Solution. Angew. Chem. Int. Ed. 2000, 46, 7659–7663. [Google Scholar] [CrossRef]
- Yu, S.H.; Cölfen, H.; Xu, A.W.; Dong, W. Complex spherical BaCO3 superstructures self-assembled by a facile mineralization process under control of simple polyelectrolytes. Cryst. Growth Des. 2004, 4, 33–37. [Google Scholar] [CrossRef]
- Yu, S.H.; Cölfen, H.; Tauer, K.; Antonietti, M. Tectonic arrangement of BaCO3 nanocrystals into helices induced by a racemic block copolymer. Nat. Mater. 2005, 4, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.P.; Fraústo da Silva, J.J.R. Bringing Chemistry to Life: From Matter to Man; Oxford University Press: New York, NY, USA, 1999; pp. 293–298. [Google Scholar]
- Fraústo da Silva, J.J.R.; Williams, R.J.P. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life, 2nd ed.; Oxford University Press: New York, NY, USA, 1991; pp. 7–10. [Google Scholar]
- Turner, D.R.; Whitfield, M.; Dickson, A.G. The Equilibrium Speciation of Dissolved Components in Fresh-Water and Seawater at 25-Degrees-C and 1 Atm Pressure. Geochim. Cosmochim. Acta 1981, 45, 855. [Google Scholar] [CrossRef]



| Type of DNA | Type of Synthesis | Raman (cm−1) | IR (cm−1) | Composition |
|---|---|---|---|---|
| Control | STP | 157, 282, 712, 1086, 1750 | 712.1, 871.1, 1399.9 | Calcite |
| Precambrian | 157, 282, 1082 | 792.0, 871.2, 950.1, 1073.3, 1387.2, 2980.7 | Calcite | |
| E. coli | STP | 157, 282, 712, 1086 | 575.3, 604.7, 712.2, 750.5, 871.7, 1407.30 | Calcite |
| Precambrian | 157, 283, 713, 1086 | 711.6, 796.6, 871.1, 1076.9, 1394.1, 2980.7, 3420.8 | Calcite | |
| E. coli + kaolinite | STP | 157, 282, 712, 1085 | 560, 757.3, 873.0, 1407.0 | Calcite |
| Precambrian | 157, 282, 713, 1086 | 544.0, 595.0, 626.0, 680.7, 797.5, 913.4, 939.0, 1008.3, 1031.0, 1386.9, 2980.7, 3482.6, 3619.6, 3690.8 | Calcite | |
| C. albicans | STP | 111, 157, 282, 470, 711, 1086, 1480, 2911 | 580, 711, 871.20, 1406.2 | Calcite, Aragonite |
| Precambrian | 114, 155, 281, 712, 1085, 1457, 1760 | 711.5, 792.6, 870.6, 1073.1, 1387.4 | Calcite, Aragonite | |
| C. albicans + kaolinite | STP | 112, 156, 281, 711, 1085, 1470, 2980 | 712.1, 757.3, 871.3, 1394.1 | Calcite, Aragonite |
| Precambrian | 112, 156, 281, 712, 1085, 1437, 1750 | 599.4, 596.0, 576.9, 626.1, 680.8, 797.4, 913.3, 939.2, 1008.2, 1031.0, 2980.8, 3691.0 | Calcite, Aragonite |
| Type of DNA | Type of Synthesis | Raman Spectroscopy (cm−1) | FTIR (cm−1) | Composition |
|---|---|---|---|---|
| Control | STP | 93, 138, 154, 221, 690, 1058 | 549.7, 585.1, 604.3, 692.6, 768.5, 855.5, 953.2, 1059.0, 1416.9, 1577.7, 2980 | Witherite |
| Precambrian | 95, 139, 155, 229, 690, 1059 | 692.8, 798.4, 855.9, 1074.0, 1418.8, 1578.9, 1749.5, 2980.8 | Witherite | |
| E. coli | STP | 93, 138, 152, 226, 690, 1058, 1412, 1508 | 563.9, 575.4, 692.4, 855.2, 954.7, 1077.3, 1417.2, 1577.4, 2980.8 | Witherite |
| Precambrian | 94, 137, 222, 690, 1058, 1354, 1497, 1503, 2195, 2801, 2938 | 576.4, 692.9, 791.6, 855.5, 945.5, 1069.7, 1420.9 | Witherite | |
| E. coli + kaolinite | STP | 93, 138, 151, 691, 1058, 1440, 2890 | 575.1, 599.3, 627.6, 692.4, 773.2, 855.5, 910.7, 1059.1, 1417.0 | Witherite |
| Precambrian | 96, 140, 222, 693, 1059, 1060, 1424, 2900 | 626.3, 692.7, 779.5, 855.8, 911.7, 1072.9, 1420.0, 2980.0 | Witherite | |
| C. albicans | STP | 93, 138, 155, 221, 689, 1058, 1356, 1503, 2840 | 692.8, 764.6, 856.1, 888.0, 1059.3, 1417.2, 1578.1, 1979.2, 2160.8, 2980.8 | Witherite |
| Precambrian | 98, 141, 225, 692, 1009, 1060, 1470, 2800 | 689.0, 764.2, 892.2, 1059.3, 1402.0, 1700.0, 2980.7 | Witherite | |
| C. albicans + kaolinite | STP | 138, 230, 693, 1059, 1425, 1504 | 595.6, 626.8, 692.4, 752.9, 856.3, 911.1, 1072.9, 1417.3, 2980.7 | Witherite |
| Precambrian | 92, 138, 153, 690, 1059, 1420 | 595.5, 626.4, 687.7, 776.6, 911.2, 1007.6, 1027.9, 2884.1, 2980.7 | Witherite |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuéllar-Cruz, M.; Islas, S.R.; Moreno, A. DNA Takes Over on the Control of the Morphology of the Composite Self-Organized Structures of Barium and Calcium Silica–Carbonate Biomorphs, Implications for Prebiotic Chemistry on Earth. Earth 2024, 5, 293-310. https://doi.org/10.3390/earth5030016
Cuéllar-Cruz M, Islas SR, Moreno A. DNA Takes Over on the Control of the Morphology of the Composite Self-Organized Structures of Barium and Calcium Silica–Carbonate Biomorphs, Implications for Prebiotic Chemistry on Earth. Earth. 2024; 5(3):293-310. https://doi.org/10.3390/earth5030016
Chicago/Turabian StyleCuéllar-Cruz, Mayra, Selene R. Islas, and Abel Moreno. 2024. "DNA Takes Over on the Control of the Morphology of the Composite Self-Organized Structures of Barium and Calcium Silica–Carbonate Biomorphs, Implications for Prebiotic Chemistry on Earth" Earth 5, no. 3: 293-310. https://doi.org/10.3390/earth5030016
APA StyleCuéllar-Cruz, M., Islas, S. R., & Moreno, A. (2024). DNA Takes Over on the Control of the Morphology of the Composite Self-Organized Structures of Barium and Calcium Silica–Carbonate Biomorphs, Implications for Prebiotic Chemistry on Earth. Earth, 5(3), 293-310. https://doi.org/10.3390/earth5030016








