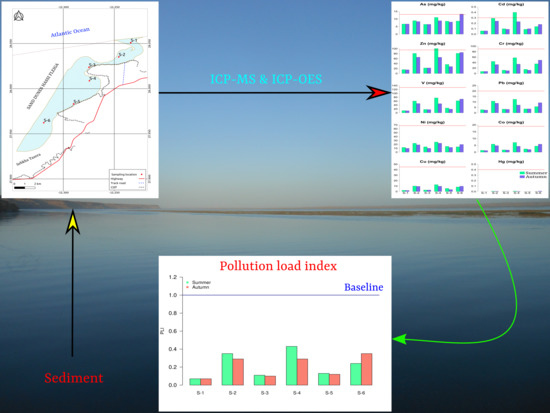
Assessment of Trace Metals in Sediments from Khnifiss Lagoon (Tarfaya, Morocco)
Abstract
:1. Introduction
2. Materials and Methods
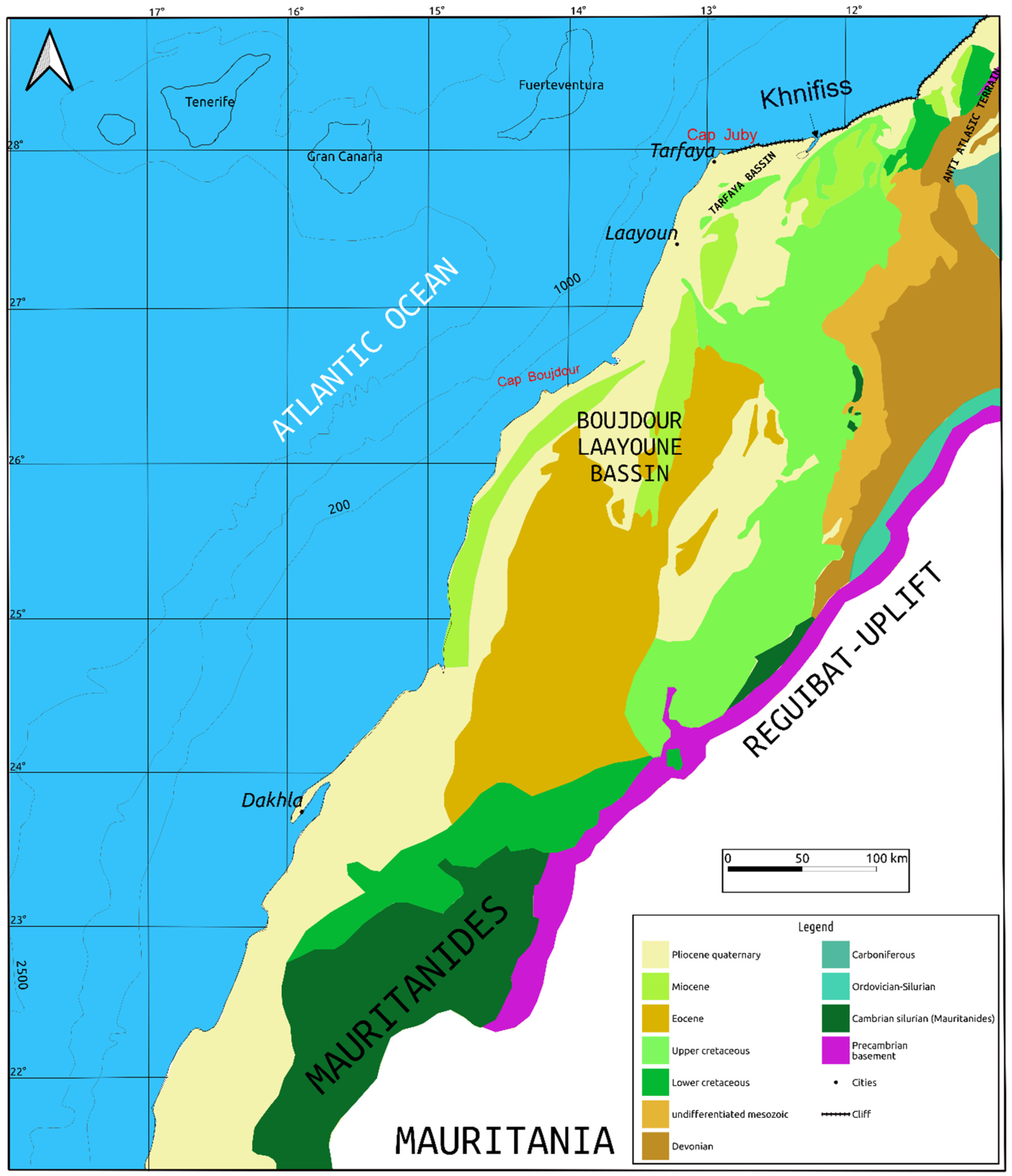
2.1. Geo−Environmental Setting of the Area
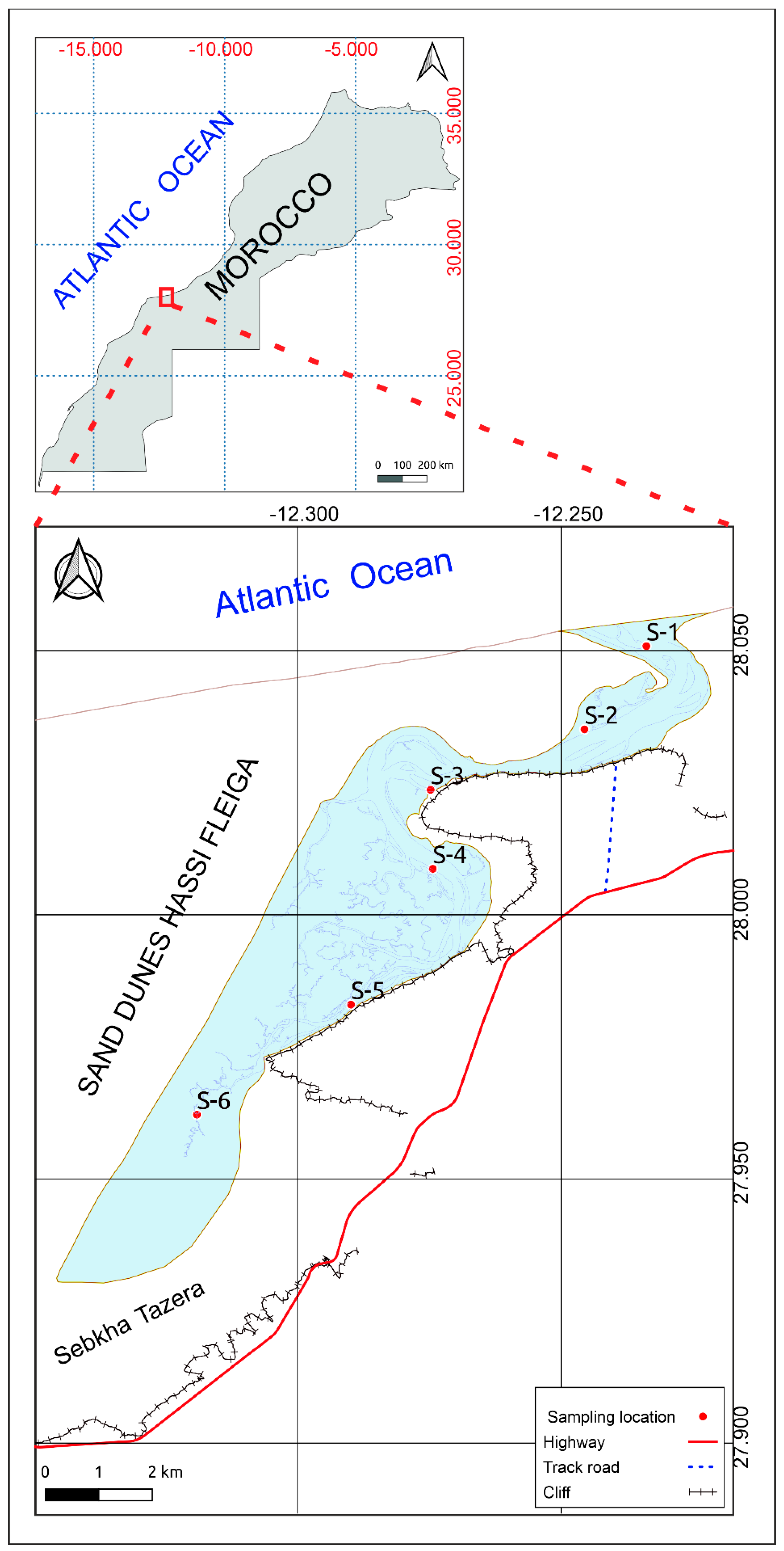
2.2. Sediment Sampling and Analysis
2.3. Pollution Indices
2.4. Correlation Analysis
3. Results and Discussion
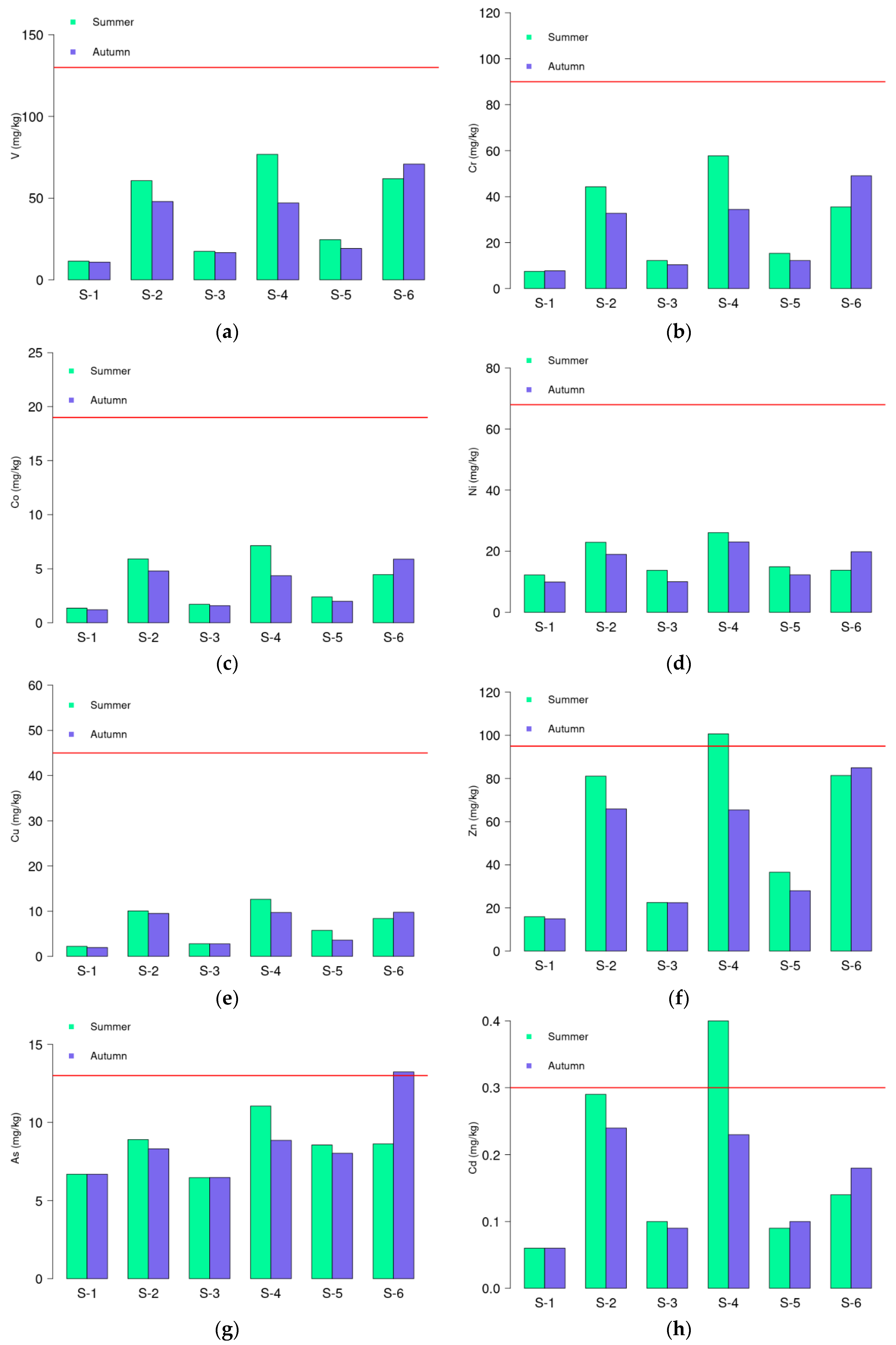
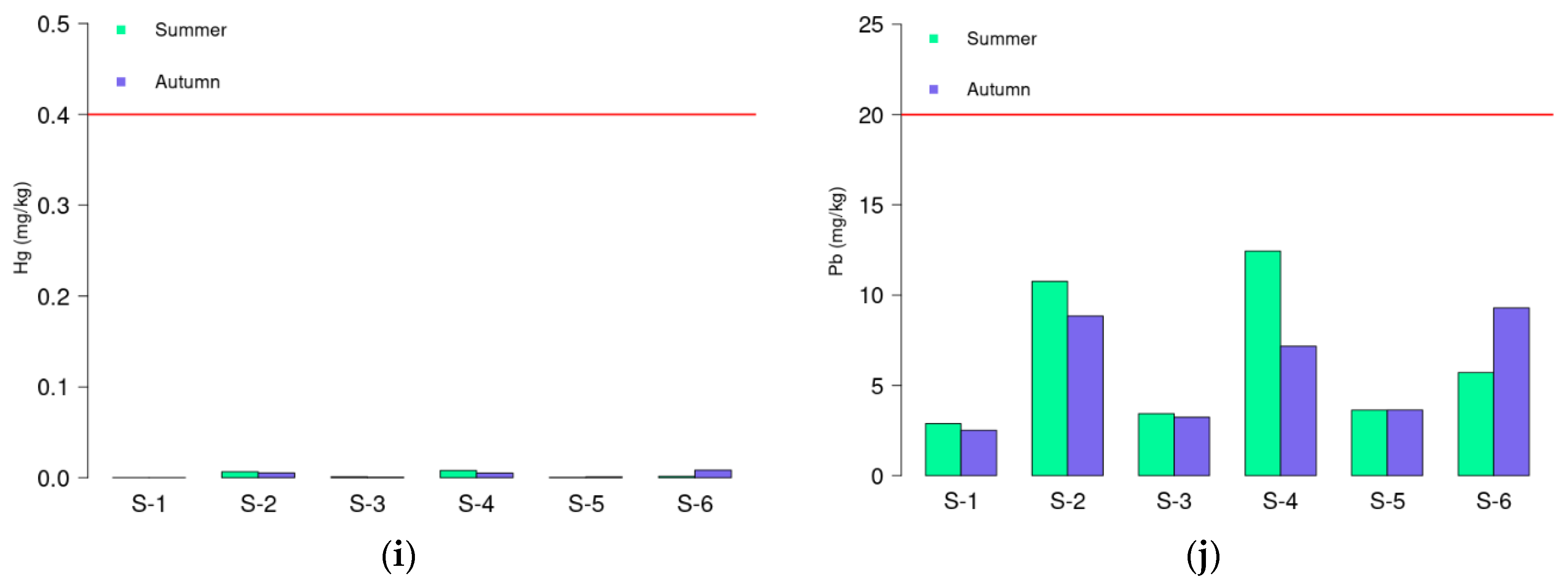
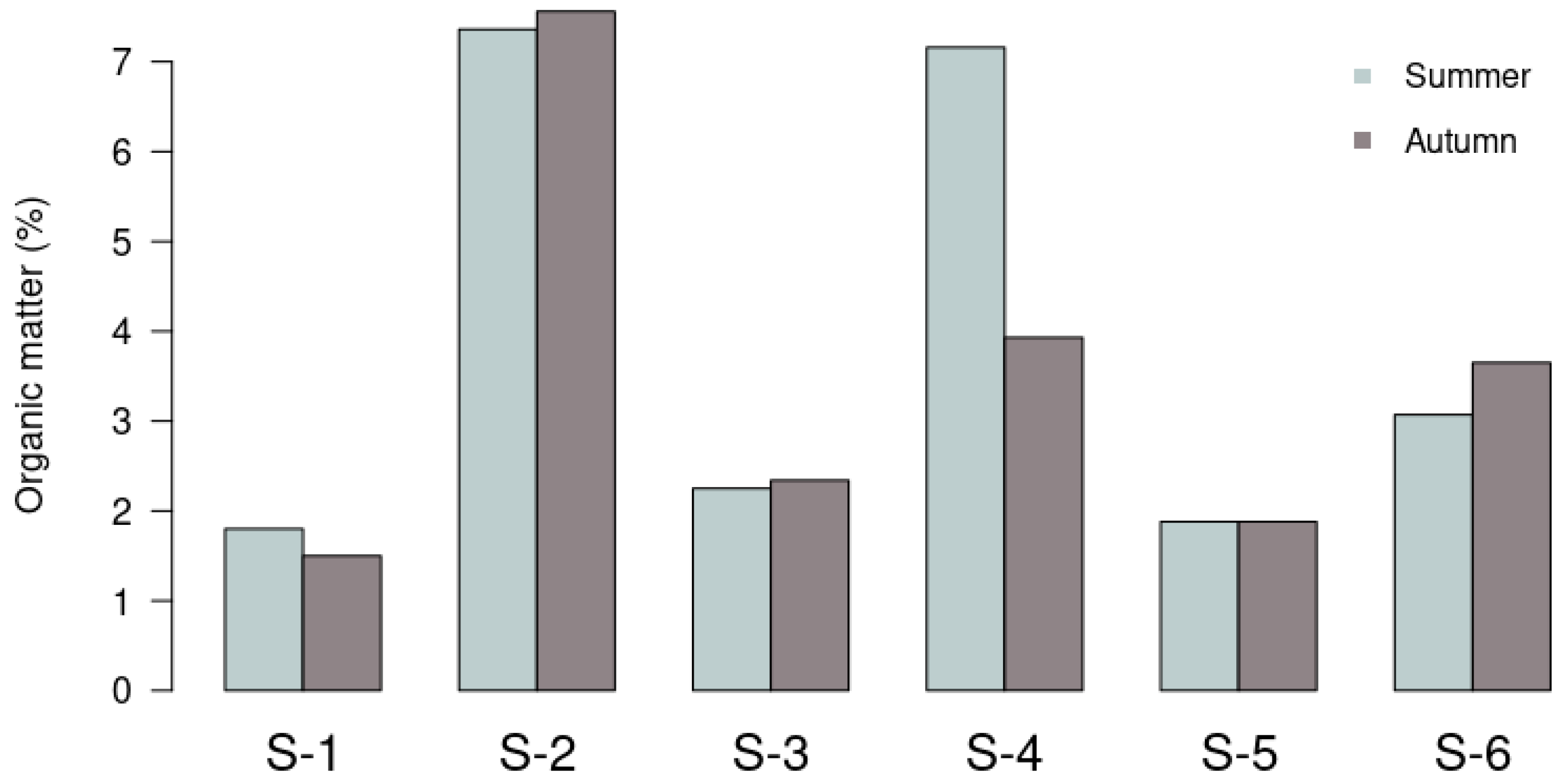
3.1. Geochemical Results
3.2. Contamination Degree Assessment
3.3. Correlation Matrix
3.4. Comparison with Other Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Van Den Broeck, M.; Waterkeyn, A.; Rhazi, L.; Grillas, P.; Brendonck, L. Assessing the ecological integrity of endorheic wetlands, with focus on Mediterranean temporary ponds. Ecol. Indic. 2015, 54, 1–11. [Google Scholar] [CrossRef]
- Zourarah, B.; Maanan, M.; Robin, M.; Carruesco, C. Sedimentary records of anthropogenic contribution to heavy metal content in Oum Er Bia estuary (Morocco). Environ. Chem. Lett. 2009, 7, 67–78. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Marcos, C.; Pérez-Ruzafa, I.M.; Pérez-Marcos, M. Coastal lagoons: “Transitional ecosystems” between transitional and coastal waters. J. Coast. Conserv. 2011, 15, 369–392. [Google Scholar] [CrossRef]
- Stanley, M.C.; Beggs, J.R.; Bassett, I.E.; Burns, B.R.; Dirks, K.N.; Jones, D.N.; Linklater, W.L.; Macinnis-Ng, C.; Simcock, R.; Souter-Brown, G.; et al. Emerging threats in urban ecosystems: A horizon scanning exercise. Front. Ecol. Environ. 2015, 13, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.; Raju, N.J.; Reddy, B.C.S.R.; Suresh, U.; Sankar, D.B.; Reddy, T.V.K. Heavy metal contamination in river water and sediments of the Swarnamukhi River Basin, India: Risk assessment and environmental implications. Environ. Geochem. Health 2018, 40, 609–623. [Google Scholar] [CrossRef]
- Armiento, G.; Caprioli, R.; Cerbone, A.; Chiavarini, S.; Crovato, C.; De Cassan, M.; De Rosa, L.; Montereali, M.R.; Nardi, E.; Nardi, L.; et al. Current status of coastal sediments contamination in the former industrial area of Bagnoli-Coroglio (Naples, Italy). Chem. Ecol. 2020. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, P.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef]
- Liu, J.; Song, J.; Yuan, H.; Li, X.; Li, N.; Duan, L. Trace metal comparative analysis of sinking particles and sediments from a coastal environment of the Jiaozhou Bay, North China: Influence from sediment resuspension. Chemosphere 2019, 232, 315–326. [Google Scholar] [CrossRef]
- Huyen, D.T.; Tabelin, C.B.; Thuan, H.M.; Dang, H.D.; Truong, P.T.; Vongphuthone, B.; Kobayashi, M.; Igarashi, T. The solid-phase partitioning of arsenic in unconsolidated sediments of the Mekong Delta, Vietnam and its modes of release under various conditions. Chemosphere 2019, 233, 512–523. [Google Scholar] [CrossRef]
- Guédron, S.; Tisserand, D.; Garambois, S.; Spadini, L.; Molton, F.; Bounvilay, B.; Charlet, L.; Polya, D.A. Baseline investigation of (methyl)mercury in waters, soils, sediments and key foodstuffs in the Lower Mekong Basin: The rapidly developing city of Vientiane (Lao PDR). J. Geochem. Explor. 2014, 143, 96–102. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Marigoudar, S.R.; Mohan, D.; Nagarjuna, A.; Sharma, K.V. Ecological risk from heavy metals in Ennore estuary, South East coast of India. Environ. Chem. Ecotoxicol. 2020, 2, 182–193. [Google Scholar] [CrossRef]
- Morillo, J.; Usero, J.; Gracia, I. Potential mobility of metals in polluted coastal sediments in two bays of southern Spain. J. Coast. Res. 2007, 23, 352–361. [Google Scholar] [CrossRef]
- Zaidi, A.; Wani, P.A.; Khan, M.S. Toxicity of heavy metals to legumes and bioremediation. In Toxicity of Heavy Metals to Legumes and Bioremediation; Springer: Vienna, Austria, 2014; pp. 29–44. [Google Scholar] [CrossRef]
- Dong, W.Q.Y.; Cui, Y.; Liu, X. Instances of soil and crop heavy metal contamination in China. Soil Sediment Contam. 2001, 10, 497–510. [Google Scholar] [CrossRef]
- Facchinelli, A.; Sacchi, E.; Mallen, L. Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environ. Pollut. 2001, 114, 313–324. [Google Scholar] [CrossRef]
- Solgi, E.; Abbas, E.S.; Alireza, R.B.; Hadipour, M. Soil contamination of metals in the three industrial estates, Arak, Iran. Bull. Environ. Contam. Toxicol. 2012, 88, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wu, K.; Li, Y.; Qi, Z.; Han, D.; Zhang, B.; Gu, C.; Chen, G.; Liu, J.; Chen, S.; et al. Blood lead and cadmium levels and relevant factors among children from an e-waste recycling town in China. Environ. Res. 2008, 108, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Amin, B.; Ismail, A.; Arshad, A.; Yap, C.K.; Kamarudin, M.S. Anthropogenic impacts on heavy metal concentrations in the coastal sediments of Dumai, Indonesia. Environ. Monit. Assess. 2009, 148, 291–305. [Google Scholar] [CrossRef]
- Wen, L.S.; Santschi, P.; Gill, G.; Paternostro, C. Estuarine trace metal distributions in Galveston Bay: Importance of colloidal forms in the speciation of the dissolved phase. Mar. Chem. 1999, 63, 185–212. [Google Scholar] [CrossRef]
- Mounier, S.; Lacerda, L.D.; Marins, R.V.; Bemaim, J. Copper and Mercury Complexing Capacity of Organic Matter from a Mangrove Mud Flat Environment, Sepetiba Bay, Brazil. Bull. Environ. Contam. Toxicol. 2001, 67, 0519–0525. [Google Scholar] [CrossRef]
- Orbi, A.; Nemmaoui, M. Fluctuation des vents et variabilité de l’upwelling le long de la Côte Atlantique Marocaine. Trav. Doc. Inst. Natl. Rech. Halieut. 1992, 75, 27. [Google Scholar]
- Lefrere, L.; Ouassas, M.; Guillois, B.; Gillet, P.; Moukrim, A. Macrobenthic community structure of soft-bottom sediments in the Khnifiss lagoon, South of Morocco. J. Mater. Environ. Sci. 2015, 6, 3226–3235. [Google Scholar]
- RGPH. Recensement Général de la Population et de l’Habitat; Royaume du Maroc: Rabat, Morocco, 2014; Available online: http://rgphentableaux.hcp.ma/ (accessed on 10 June 2020).
- Beaubrun, P.C. La lagune de Khnifiss: Premières observations sur les sédiments et l’hydrologie du milieu. Bull. Inst. Sci 1976, 1, 50–65. [Google Scholar]
- Lakhdar Idrissi, J.; Orbi, A.; Zidane, F.; Hilmi, K.; Sarf, F.; Massik, Z.; Makaoui, A. Organisation et fonctionnement d’un écosystème côtier du Maroc: La lagune de Khnifiss. Rev. Sci. L’eau 2004, 17, 447–462. [Google Scholar] [CrossRef] [Green Version]
- El Agbani, M.A.; Fekhaoui, M.; Bayed, A.; Schouten, J. The Khnifiss lagoon and adjacent waters: Hydrology and hydrodynamics. In The Khnifiss Lagoon and Its Surrounding Environment (Province of La’youne, Morocco); Dakki, M., de Ligny, W., Eds.; Travaux de l’Institut Scientifique: Rabat, Morocco, 1988; pp. 17–26. [Google Scholar]
- Hassi, M.; Alouani, M.; Faaras, M.S.; Staiti, M. Qualitative and quantitative study of the spring phytoplankton community in the Naïla lagoon (Moroccan Atlantic coast). J. Mater. Environ. Sci. 2020, 11, 977–987. [Google Scholar]
- Edmondson, J.; Gunn, A.; Burt, A.J.; Parker, D.M. Checklist of Flora of the Khnifiss-Tarfaya region (Morocco). In The Khnifiss Lagoon and Its Surrounding Environment (Province of La’youne, Morocco); Dakki, M., de Ligny, W., Eds.; Travaux de l’Institut Scientifique: Rabat, Morocco, 1988; pp. 41–45. [Google Scholar]
- Russel, G.; Hockin, D.C. The Seaweeds of the Khnifiss lagoon and the Tarfaya coast. In The Khnifiss Lagoon and Its Surrounding Environment (Province of La’youne, Morocco); Dakki, M., de Ligny, W., Eds.; Travaux de l’Institut Scientifique: Rabat, Morocco, 1988; pp. 37–40. [Google Scholar]
- Dillon, W.P.; Sougy, J.M.A. Geology of West Africa and Canary and Cape Verde Islands; Springer Science and Business Media LLC: Boston, MA, USA, 1974; pp. 315–390. [Google Scholar]
- Choubert, G.; Faure-Muret, A.; Hottinger, L.; Viotti, G.; Lecointre, G. Le bassin côtier de Tarfaya (Maroc méridional). Notes Mém. Serv. Géol. Maroc. 1966, 175, 1–285. [Google Scholar]
- Michard, A.; Saddiqi, O.; Chalouan, A.; de Lamotte, D.F. (Eds.) Continental Evolution: The Geology of Morocco; Lecture Notes in Earth Sciences; Springer: Berlin/Heidelberg, Germany, 2008; Volume 116, ISBN 978-3-540-77075-6. [Google Scholar]
- Ranke, U.; von Rad, U.; Wissmann, G. Stratigraphy, facies and tectonic development of the on-and offshore Aaiun-Tarfaya Basin—A review. In Geology of the Northwest African Continental Margin; Springer: Berlin/Heidelberg, Germany, 1982; pp. 86–105. [Google Scholar]
- Gilli, R.; Karlen, C.; Weber, M.; Rüegg, J.; Barmettler, K.; Biester, H.; Boivin, P.; Kretzschmar, R. Speciation and Mobility of Mercury in Soils Contaminated by Legacy Emissions from a Chemical Factory in the Rhône Valley in Canton of Valais, Switzerland. Soil Syst. 2018, 2, 44. [Google Scholar] [CrossRef] [Green Version]
- Franzen, C.; Kilian, R.; Biester, H. Natural mercury enrichment in a minerogenic fen—Evaluation of sources and processes. J. Environ. Monit. 2004, 6, 466–472. [Google Scholar] [CrossRef]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Lamas, F.; Irigaray, C.; Oteo, C.; Chacón, J. Selection of the most appropriate method to determine the carbonate content for engineering purposes with particular regard to marls. Eng. Geol. 2005, 81, 32–41. [Google Scholar] [CrossRef]
- Valdés, J.; Vargas, G.; Sifeddine, A.; Ortlieb, L.; Guiñez, M. Distribution and enrichment evaluation of heavy metals in Mejillones Bay (23°S), Northern Chile: Geochemical and statistical approach. Mar. Pollut. Bull. 2005, 50, 1558–1568. [Google Scholar] [CrossRef]
- Rowell, D. Soil Science: Methods & Applications; Longman Scientific & Technical: London, UK, 1994. [Google Scholar]
- Muller, G. Methods in sedimentary petrology. In Sedimentary Petrology (Pt. I); Engelhardt, W.V., Fiichtbauer, H., Muller, G., Eds.; Schweitzerbartsche Verlagsbuchhandlung: Stuttgart, Germany, 1967. [Google Scholar]
- Bao, K.; Jia, L.; Lu, X.; Wang, G. Grain-size characteristics of sediment in Daniugou Peatland in Changbai Mountains, Northeast China: Implications for atmospheric dust deposition. Chin. Geogr. Sci. 2010, 20, 498–505. [Google Scholar] [CrossRef] [Green Version]
- Tabelin, C.B.; Silwamba, M.; Paglinawan, F.C.; Mondejar, A.J.S.; Duc, H.G.; Resabal, V.J.; Opiso, E.M.; Igarashi, T.; Tomiyama, S.; Ito, M.; et al. Solid-phase partitioning and release-retention mechanisms of copper, lead, zinc and arsenic in soils impacted by artisanal and small-scale gold mining (ASGM) activities. Chemosphere 2020, 260, 127574. [Google Scholar] [CrossRef] [PubMed]
- Bantan, R.A.; Al-Dubai, T.A.; Al-Zubieri, A.G. Geo-environmental assessment of heavy metals in the bottom sediments of the Southern Corniche of Jeddah, Saudi Arabia. Mar. Pollut. Bull. 2020, 161, 111721. [Google Scholar] [CrossRef] [PubMed]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the Elements in Some Major Units of the Earth’s Crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Loska, K.; Cebula, J.; Pelczar, J.; Wiechuła, D.; Kwapuliński, J. Use of enrichment, and contamination factors together with geoaccumulation indexes to evaluate the content of Cd, Cu, and Ni in the Rybnik water Reservoir in Poland. Water Air Soil Pollut. 1997, 93, 347–365. [Google Scholar] [CrossRef]
- Mata, H.K.; Al Salah, D.M.M.; Ngweme, G.N.; Konde, J.N.; Mulaji, C.K.; Kiyombo, G.M.; Poté, J.W. Toxic metal concentration and ecotoxicity test of sediments from dense populated areas of Congo River, Kinshasa, Democratic Republic of the Congo. Environ. Chem. Ecotoxicol. 2020, 2, 83–90. [Google Scholar] [CrossRef]
- Liaghati, T.; Preda, M.; Cox, M. Heavy metal distribution and controlling factors within coastal plain sediments, Bells Creek catchment, southeast Queensland, Australia. Environ. Int. 2004, 29, 935–948. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, L.; Pan, B.; Guan, W.; Sun, X.; Cai, A. Chemosphere Distribution features and controls of heavy metals in surface sediments from the riverbed of the Ningxia-Inner Mongolian reaches, Yellow River, China. Chemosphere 2016, 144, 29–42. [Google Scholar] [CrossRef]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. Geol. J. 1969, 2, 109–118. [Google Scholar]
- Zhang, W.; Feng, H.; Chang, J.; Qu, J.; Xie, H.; Yu, L. Heavy metal contamination in surface sediments of Yangtze River intertidal zone: An assessment from different indexes. Environ. Pollut. 2009, 157, 1533–1543. [Google Scholar] [CrossRef]
- Chakraborty, P.; Ramteke, D.; Chakraborty, S.; Nagender Nath, B. Changes in metal contamination levels in estuarine sediments around India—An assessment. Mar. Pollut. Bull. 2014, 78, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Buccolieri, A.; Buccolieri, G.; Cardellicchio, N.; Dell’Atti, A.; Di Leo, A.; Maci, A. Heavy metals in marine sediments of Taranto Gulf (Ionian Sea, Southern Italy). Mar. Chem. 2006, 99, 227–235. [Google Scholar] [CrossRef]
- Gong, Q.; Deng, J.; Xiang, Y.; Wang, Q.; Yang, L. Calculating Pollution Indices by Heavy Metals in Ecological Geochemistry Assessment and a Case Study in Parks of Beijing. J. China Univ. Geosci. 2008, 19, 230–241. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.r-project.org/ (accessed on 13 May 2020).
- Leenheer, J.A. Analysis, association, and effects of organic constituents of aquatic constituents sediments. In The Role of Sediments in the Chemistry of Aquatic Systems; Bradford, L., Horowitz, J.A., Eds.; U.S. Geo-logical Survey Circular 969; U.S. Government Printing Office: Washington, DC, USA, 1982; pp. 22–32. [Google Scholar]
- Alaoui, A.M.; Choura, M.; Maanan, M.; Zourarah, B.; Robin, M.; Conceição, M.F.; Andrade, C.; Khalid, M.; Carruesco, C. Metal fluxes to the sediments of the Moulay Bousselham lagoon, Morocco. Environ. Earth Sci. 2010, 61, 275–286. [Google Scholar] [CrossRef]
- Zourarah, B.; Maanan, M.; Carruesco, C.; Aajjane, A.; Mehdi, K.; Conceição Freitas, M. Fifty-year sedimentary record of heavy metal pollution in the lagoon of Oualidia (Moroccan Atlantic coast). Estuar. Coast. Shelf Sci. 2007, 72, 359–369. [Google Scholar] [CrossRef]
- Villalobos, M.; Leckie, J. The role of carbonate in trace metal soil pollution. Rev. Int. Contam. Ambient 2000, 16, 157–167. [Google Scholar]
- Drever, J.I. The Geochemistry of Natural Waters, 2nd ed.; Prentice-Hall: New York, NY, USA, 2008. [Google Scholar]
- Callender, E. Heavy metals in the environment—Historical trends. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 59–89. [Google Scholar]
- Birch, G.F. Determination of sediment metal background concentrations and enrichment in marine environments—A critical review. Sci. Total Environ. 2017, 580, 813–831. [Google Scholar] [CrossRef]
- Idardare, Z.; Chiffoleau, J.-F.; Moukrim, A.; Alla, A.A.; Auger, D.; Lefrere, L.; Rozuel, E. Metal concentrations in sediment andNereis diversicolorin two Moroccan lagoons: Khnifiss and Oualidia. Chem. Ecol. 2008, 24, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Leine, L. Geology of the Tarfaya oil shale deposit, Morocco. Geol. Mijnb. 1986, 65, 57–74. [Google Scholar]
- Atgin, S.R.; El-Agha, O.; Zararsiz, A.; Kocataş, A.; Parlak, H.; Tuncel, G. Investigation of the sediment pollution in Izmir Bay: Trace elements. Spectrochim. Acta Part B At. Spectrosc. 2000, 55, 1151–1164. [Google Scholar] [CrossRef]
- Bloundi, M.K.; Duplay, J.; Quaranta, G. Heavy metal contamination of coastal lagoon sediments by anthropogenic activities: The case of Nador (East Morocco). Environ. Geol. 2009, 56, 833–843. [Google Scholar] [CrossRef]
- Rumisha, C.; Elskens, M.; Leermakers, M.; Kochzius, M. Trace metal pollution and its influence on the community structure of soft bottom molluscs in intertidal areas of the Dar es Salaam coast, Tanzania. Mar. Pollut. Bull. 2012, 64, 521–531. [Google Scholar] [CrossRef]
- Acevedo-Figueroa, D.; Jiménez, B.D.; Rodríguez-Sierra, C.J. Trace metals in sediments of two estuarine lagoons from Puerto Rico. Environ. Pollut. 2006, 141, 336–342. [Google Scholar] [CrossRef]
- Agah, H.; Saleh, A.; Bastami, K.D.; Fumani, N.S. Ecological risk, source and preliminary assessment of metals in the surface sediments of Chabahar Bay, Oman Sea. Mar. Pollut. Bull. 2016, 107, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.; El-Sorogy, A. Environmental assessment of heavy metal contamination in bottom sediments of Al-Kharrar lagoon, Rabigh, Red Sea, Saudi Arabia. Arab. J. Geosci. 2016, 9, 474. [Google Scholar] [CrossRef]
- Mejjad, N.; Laissaoui, A.; El-Hammoumi, O.; Fekri, A.; Amsil, H.; El-Yahyaoui, A.; Benkdad, A. Geochemical, radiometric, and environmental approaches for the assessment of the intensity and chronology of metal contamination in the sediment cores from Oualidia lagoon (Morocco). Environ. Sci. Pollut. Res. 2018, 25, 22872–22888. [Google Scholar] [CrossRef]
- Hossain, M.B.; Shanta, T.B.; Ahmed, A.S.S.; Hossain, M.K.; Semme, S.A. Baseline study of heavy metal contamination in the Sangu River estuary, Chattogram, Bangladesh. Mar. Pollut. Bull. 2019, 140, 255–261. [Google Scholar] [CrossRef]
- Kükrer, S.; Erginal, A.E.; Kılıç, Ş.; Bay, Ö.; Akarsu, T.; Öztura, E. Ecological risk assessment of surface sediments of Çardak Lagoon along a human disturbance gradient. Environ. Monit. Assess. 2020, 192. [Google Scholar] [CrossRef]
- Birch, G.; Lee, J.H. The use of sedimentary metal data in predictive modelling of estuarine contamination, assessment of environmental condition and pollutant source identification (Narrabeen Lagoon, Sydney, Australia). Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
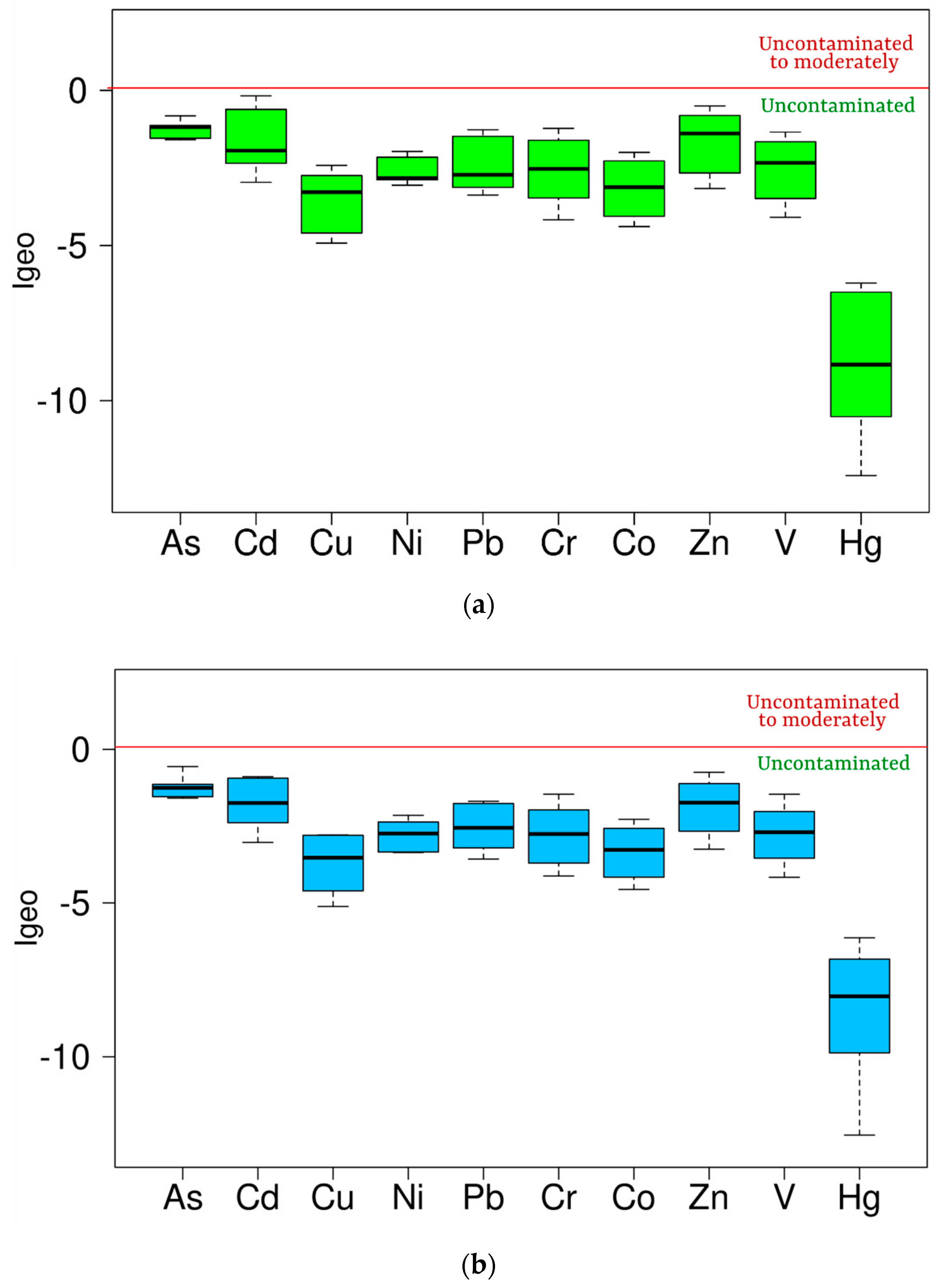
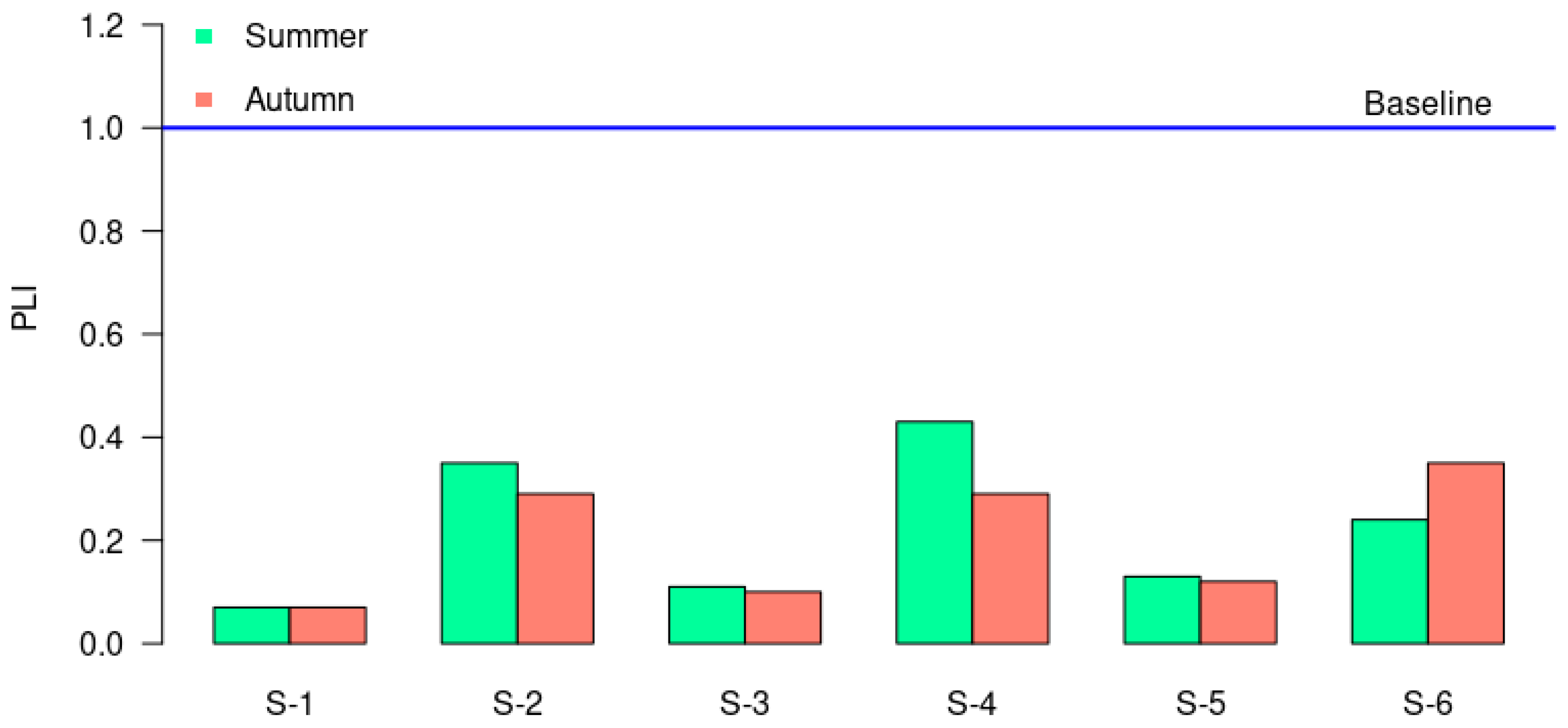
| PLI [53] | Degree of Contamination | Igeo [54] | Degree of Contamination | EF [55] | Degree of Contamination |
|---|---|---|---|---|---|
| <1 | Unpolluted | Igeo ≤ 0 | Uncontaminated | EF < 2 | No enrichment/Depletion to mineral |
| >1 | Polluted | 0 < Igeo ≤ 1 | Uncontaminated to moderately | 2 ≤ EF< 5 | Moderate enrichment |
| 1 < Igeo ≤ 2 | Moderately contaminated | 5 ≤ EF < 20 | Significant enrichment | ||
| 2 < Igeo ≤ 3 | Moderately to highly contaminated | 20 ≤ EF < 40 | Very high enrichment | ||
| 3 < Igeo ≤ 4 | Highly contaminated | EF ≥ 40 | Extremely enrichment | ||
| 4 < Igeo ≤ 5 | Highly to extremely contaminated | ||||
| Igeo > 5 | Extremely contaminated |
| Al % | V mg/kg | Cr mg/kg | Co mg/kg | Ni mg/kg | Cu mg/kg | Zn mg/kg | As mg/kg | Cd mg/kg | Hg mg/kg | Pb mg/kg | pH | OM % | CaCO3 % | Clay % | Silt % | Sand % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S−1 | 1.79 | 11. 5 | 7.50 | 1.36 | 12.3 | 2.23 | 15.9 | 6.70 | 0.06 | <0.005 | 2.89 | 8.20 | 1.80 | 54.5 | 0.03 | 0.80 | 99.2 |
| S−2 | 4.05 | 60.7 | 44.3 | 5.90 | 22.9 | 10.1 | 81.1 | 8.92 | 0.29 | 0.01 | 10.8 | 7.68 | 7.36 | 29.6 | 20.4 | 45.5 | 34.1 |
| S−3 | 1.46 | 17.4 | 12.2 | 1.71 | 13.8 | 2.79 | 22.5 | 6.48 | 0.10 | <0.005 | 3.44 | 7.89 | 2.25 | 51.9 | 1.80 | 2.80 | 95.4 |
| S−4 | 4.78 | 76.8 | 57.8 | 7.13 | 26.1 | 12.6 | 101 | 11.1 | 0.40 | 0.01 | 12.4 | 7.59 | 7.16 | 13.3 | 22.4 | 58.6 | 19.1 |
| S−5 | 2.10 | 24.6 | 15.4 | 2.40 | 15.0 | 5.77 | 36.6 | 8.57 | 0.09 | <0.005 | 3.63 | 8.27 | 1.88 | 33.8 | 0.26 | 1.10 | 98.6 |
| S−6 | 2.72 | 61.9 | 35.5 | 4.47 | 13.8 | 8.38 | 81.4 | 8.64 | 0.14 | <0.005 | 5.71 | 7.82 | 3.07 | 22.4 | 5.54 | 7.82 | 86.6 |
| S−1 | 1.45 | 10.9 | 7.74 | 1.21 | 9.95 | 1.95 | 15.0 | 6.70 | 0.06 | <0.005 | 2.52 | 8.24 | 1.50 | 58.8 | 0.24 | 0.62 | 99.1 |
| S−2 | 3.47 | 48.0 | 32.8 | 4.80 | 19.0 | 9.50 | 65.9 | 8.33 | 0.24 | 0.01 | 8.85 | 7.55 | 7.56 | 28.4 | 16.3 | 37.4 | 46.3 |
| S−3 | 1.56 | 16.7 | 10.4 | 1.59 | 10.1 | 2.77 | 22.5 | 6.49 | 0.09 | <0.005 | 3.24 | 7.76 | 2.34 | 45.1 | 0.51 | 2.50 | 97.0 |
| S−4 | 3.41 | 47.1 | 34.5 | 4.36 | 23.0 | 9.71 | 65.4 | 8.87 | 0.23 | 0.01 | 7.17 | 7.93 | 3.93 | 23.8 | 10.3 | 21.4 | 68.3 |
| S−5 | 1.78 | 19.2 | 12.2 | 1.99 | 12.3 | 3.60 | 28.0 | 8.04 | 0.10 | <0.005 | 3.64 | 7.88 | 1.88 | 32.6 | 0.10 | 0.80 | 99.1 |
| S−6 | 4.04 | 70.8 | 49.1 | 5.88 | 19.8 | 9.77 | 85.0 | 13.2 | 0.18 | 0.01 | 9.29 | 7.72 | 4.68 | 8.25 | 29.4 | 29.3 | 41.3 |
| Minimum | 1.45 | 10.9 | 7.50 | 1.21 | 9.95 | 1.95 | 15.0 | 6.48 | 0.06 | 0.01 | 2.52 | 7.55 | 1.50 | 8.25 | 0.03 | 0.62 | 19.1 |
| Maximum | 4.78 | 76.8 | 57.8 | 7.13 | 26.1 | 12.6 | 101 | 13.2 | 0.40 | 0.01 | 12.4 | 8.27 | 7.56 | 58.8 | 29.4 | 58.6 | 99.2 |
| Mean | 2.72 | 38.8 | 26.6 | 3.57 | 16.5 | 6.60 | 51.7 | 8.50 | 0.16 | 0.01 | 6.13 | 7.88 | 3.78 | 33.6 | 8.94 | 17.4 | 73.7 |
| St.dev. | 1.18 | 24.7 | 17.8 | 2.09 | 5.47 | 3.81 | 31.3 | 2.00 | 0.11 | 0.00 | 3.46 | 0.25 | 2.35 | 16.1 | 10.6 | 20.6 | 30.4 |
| 1 Quartile | 1.72 | 17.3 | 11.7 | 1.68 | 12.3 | 2.78 | 22.5 | 6.70 | 0.09 | 0.01 | 3.39 | 7.71 | 1.88 | 23.5 | 0.26 | 1.03 | 45.1 |
| 3 Quartile | 3.61 | 61.0 | 37.7 | 5.07 | 20.6 | 9.73 | 81.2 | 8.88 | 0.24 | 0.01 | 8.96 | 8.00 | 5.30 | 46.8 | 17.3 | 31.3 | 98.8 |
| Ref. data: Average Shale | 8 | 130 | 90.0 | 19.0 | 68.0 | 45.0 | 95.0 | 13.0 | 0.30 | 0.40 | 20.0 | - | - | - | - | - | - |
| V | Cr | Co | Ni | Cu | Zn | As | Cd | Hg | Pb | |
|---|---|---|---|---|---|---|---|---|---|---|
| S−1 | 0.39 | 0.37 | 0.32 | 0.81 | 0.22 | 0.75 | 2.30 | 0.86 | 0 | 0.65 |
| S−2 | 0.92 | 0.97 | 0.61 | 0.67 | 0.44 | 1.69 | 1.36 | 1.94 | 0.03 | 1.06 |
| S−3 | 0.73 | 0.74 | 0.49 | 1.11 | 0.34 | 1.30 | 2.73 | 1.73 | 0.01 | 0.94 |
| S−4 | 0.99 | 1.07 | 0.63 | 0.64 | 0.47 | 1.78 | 1.42 | 2.22 | 0.03 | 1.04 |
| S−5 | 0.72 | 0.65 | 0.48 | 0.84 | 0.49 | 1.47 | 2.51 | 1.12 | 0 | 0.69 |
| S−6 | 1.40 | 1.16 | 0.69 | 0.60 | 0.55 | 2.52 | 1.95 | 1.41 | 0.01 | 0.84 |
| S−1 | 0.46 | 0.48 | 0.35 | 0.81 | 0.24 | 0.87 | 2.85 | 1.02 | 0 | 0.70 |
| S−2 | 0.85 | 0.84 | 0.58 | 0.64 | 0.49 | 1.60 | 1.48 | 1.87 | 0.03 | 1.02 |
| S−3 | 0.66 | 0.59 | 0.43 | 0.76 | 0.32 | 1.21 | 2.56 | 1.47 | 0.01 | 0.83 |
| S−4 | 0.85 | 0.90 | 0.54 | 0.79 | 0.51 | 1.61 | 1.60 | 1.84 | 0.03 | 0.84 |
| S−5 | 0.66 | 0.61 | 0.47 | 0.81 | 0.36 | 1.32 | 2.78 | 1.49 | 0.01 | 0.82 |
| S−6 | 1.08 | 1.08 | 0.61 | 0.58 | 0.43 | 1.77 | 2.02 | 1.19 | 0.04 | 0.92 |
| Average | 0.81 | 0.79 | 0.52 | 0.75 | 0.40 | 1.49 | 2.13 | 1.51 | 0.02 | 0.86 |
| Al | V | Cr | Co | Ni | Cu | Zn | As | Cd | Hg | Pb | pH | OM | CaCO3 | Clay | Silt | Sand | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | - | 0.66 | 0.66 | 0.66 | −0.77 | 0.49 | 0.77 | −0.89 | 0.66 | −0.83 | 0.60 | −0.77 | 0.77 | −0.89 | 0.83 | 0.83 | −0.83 |
| V | 1.00 | - | 1.00 | 1.00 | −0.77 | 0.66 | 0.94 | −0.54 | 0.66 | −0.62 | 0.60 | −0.77 | 0.77 | −0.89 | 0.83 | 0.83 | −0.83 |
| Cr | 0.94 | 0.94 | - | 1.00 | −0.77 | 0.66 | 0.94 | −0.54 | 0.66 | −0.62 | 0.60 | −0.77 | 0.77 | −0.89 | 0.83 | 0.83 | −0.83 |
| Co | 1.00 | 1.00 | 0.94 | - | −0.77 | 0.66 | 0.94 | −0.54 | 0.66 | −0.62 | 0.60 | −0.77 | 0.77 | −0.89 | 0.83 | 0.83 | −0.83 |
| Ni | −0.71 | −0.71 | −0.60 | −0.71 | - | −0.54 | −0.83 | 0.77 | −0.31 | 0.62 | −0.26 | 0.66 | −0.54 | 0.77 | −0.60 | −0.60 | 0.60 |
| Cu | 0.77 | 0.77 | 0.83 | 0.77 | −0.37 | - | 0.83 | −0.20 | 0.14 | −0.83 | 0.09 | −0.14 | 0.31 | −0.71 | 0.37 | 0.37 | −0.37 |
| Zn | 0.94 | 0.94 | 1.00 | 0.94 | −0.60 | 0.83 | - | −0.60 | 0.54 | −0.83 | 0.49 | −0.66 | 0.71 | −0.94 | 0.77 | 0.77 | −0.77 |
| As | −0.77 | −0.77 | −0.71 | −0.77 | 0.66 | −0.89 | −0.71 | - | −0.54 | 0.62 | −0.60 | 0.77 | −0.77 | 0.66 | −0.71 | −0.71 | 0.71 |
| Cd | 0.24 | 0.24 | 0.24 | 0.24 | 0.00 | 0.72 | 0.24 | −0.72 | - | −0.41 | 0.94 | −0.89 | 0.89 | −0.71 | 0.94 | 0.94 | −0.94 |
| Hg | −0.88 | −0.88 | −0.88 | −0.88 | 0.68 | −0.88 | −0.88 | 0.88 | −0.41 | - | −0.41 | 0.41 | −0.62 | 0.83 | −0.62 | −0.62 | 0.62 |
| Pb | 0.89 | 0.89 | 0.77 | 0.89 | −0.83 | 0.77 | 0.77 | −0.94 | 0.48 | −0.88 | - | −0.83 | 0.94 | −0.60 | 0.89 | 0.89 | −0.89 |
| pH | −0.71 | −0.71 | −0.49 | −0.71 | 0.77 | −0.37 | −0.49 | 0.66 | −0.24 | 0.49 | −0.83 | - | −0.89 | 0.77 | −0.94 | −0.94 | 0.94 |
| OM | 0.89 | 0.89 | 0.77 | 0.89 | −0.83 | 0.77 | 0.77 | −0.94 | 0.48 | −0.88 | 1.00 | −0.83 | - | −0.77 | 0.94 | 0.94 | −0.94 |
| CaCO3 | −0.94 | −0.94 | −1.00 | −0.94 | 0.60 | −0.83 | −1.00 | 0.71 | −0.24 | 0.88 | −0.77 | 0.49 | −0.77 | - | −0.89 | −0.89 | 0.89 |
| Clay | 0.83 | 0.83 | 0.77 | 0.83 | −0.94 | 0.60 | 0.77 | −0.77 | 0.12 | −0.88 | 0.89 | −0.66 | 0.89 | −0.77 | - | 1.00 | −1.00 |
| Silt | 0.89 | 0.89 | 0.77 | 0.89 | −0.83 | 0.77 | 0.77 | −0.94 | 0.48 | −0.88 | 1.00 | −0.83 | 1.00 | −0.77 | 0.89 | - | −1.00 |
| Sand | −0.94 | −0.94 | −0.89 | −0.94 | 0.89 | −0.71 | −0.89 | 0.83 | −0.24 | 0.88 | −0.94 | 0.77 | −0.94 | 0.89 | −0.94 | −0.94 | - |
| Country | Location | V | Cr | Co | Ni | Cu | Zn | As | Cd | Hg | Pb | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morocco | Khnifiss Lagoon | 10.9–76.8 (38.8) | 7.50–57.8 (26.6) | 1.21–7.13 (3.57) | 9.95–26.1 (16.5) | 1.95–12.6 (6.60) | 15.0–101 (51.7) | 6.48–13.2 (8.50) | 0.06–0.40 (0.16) | <0.005–0.01 (0.01) | 2.52–12.4 (6.13) | This study |
| Khnifiss Lagoon | - | 62 | - | 35 | 17 | 70 | - | 0.37 | - | 6 | [64] | |
| Moulay Bousselham Lagoon | - | 19–113 | - | 11–96 | 22–311 | 167–759 | - | 0.02–0.84 | 0.02–0.61 | 6.2–32 | [58] | |
| Oualidia Lagoon | - | 52.48 | - | - | 36.46 | 227.86 | - | - | 0.66 | 54.59 | [59] | |
| Nador Lagoon | 85 | 55 | 15 | 26 | 36 | 98 | 14 | 1 | - | 52 | [67] | |
| Oualidia Lagoon | 76.9 | 102.4 | 13.1 | 15.73 | 17.7 | 75.8 | 10.9 | 0.66 | - | 10.1 | [72] | |
| Italy | Bagnoli | 100 | 39.8 | - | 12.6 | 26.4 | 287 | 41.6 | 0.46 | 0.35 | 121 | [7] |
| Tanzania | Dar es Salaam Coast | 4.55 | 4.28 | 0.76 | 1.12 | 0.83 | 5.75 | 0.71 | 0.217 | - | 1.21 | [68] |
| Puerto Rico | Joyuda Lagoon | - | - | - | - | 22 | 52 | 18 | 0.10 | 0.17 | 7.6 | [69] |
| San Jose Lagoon | - | - | - | - | 105 | 531 | 13.3 | 1.8 | 1.9 | 219 | [69] | |
| Iran | Chabahar Bay, Oman Sea | 47.21 | 185.01 | 8.91 | 40.51 | 14.16 | 37.86 | 13.22 | - | - | 12.87 | [70] |
| Saudi Arabia | Al Kharrar Lagoon | - | 20.62 | 4.77 | 8.67 | 16 | 39.71 | 1.67 | 0.26 | 0.01 | 50.87 | [71] |
| Bangladesh | Sangu River Estuary | - | 25.15 | - | 32.75 | 29.24 | 88.97 | 2.58 | - | - | 19.58 | [73] |
| Turkey | Çardak Lagoon | 43.6 | 55.6 | 12.0 | 57.9 | 32.9 | 65.3 | 18.6 | 0.2 | 0.046 | 18.6 | [74] |
| Australia | Narrabeen Lagoon | - | 21 | 5.0 | 11 | 16 | 103 | - | - | - | 47 | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tnoumi, A.; Angelone, M.; Armiento, G.; Caprioli, R.; Crovato, C.; De Cassan, M.; Montereali, M.R.; Nardi, E.; Parrella, L.; Proposito, M.; et al. Assessment of Trace Metals in Sediments from Khnifiss Lagoon (Tarfaya, Morocco). Earth 2021, 2, 16-31. https://doi.org/10.3390/earth2010002
Tnoumi A, Angelone M, Armiento G, Caprioli R, Crovato C, De Cassan M, Montereali MR, Nardi E, Parrella L, Proposito M, et al. Assessment of Trace Metals in Sediments from Khnifiss Lagoon (Tarfaya, Morocco). Earth. 2021; 2(1):16-31. https://doi.org/10.3390/earth2010002
Chicago/Turabian StyleTnoumi, Ali, Massimo Angelone, Giovanna Armiento, Raffaela Caprioli, Cinzia Crovato, Maurizio De Cassan, Maria Rita Montereali, Elisa Nardi, Luisa Parrella, Marco Proposito, and et al. 2021. "Assessment of Trace Metals in Sediments from Khnifiss Lagoon (Tarfaya, Morocco)" Earth 2, no. 1: 16-31. https://doi.org/10.3390/earth2010002