Assessment of Potential Environmental Risks Posed by Soils of a Deactivated Coal Mining Area in Northern Portugal—Impact of Arsenic and Antimony
Abstract
1. Introduction
2. Experimental Part
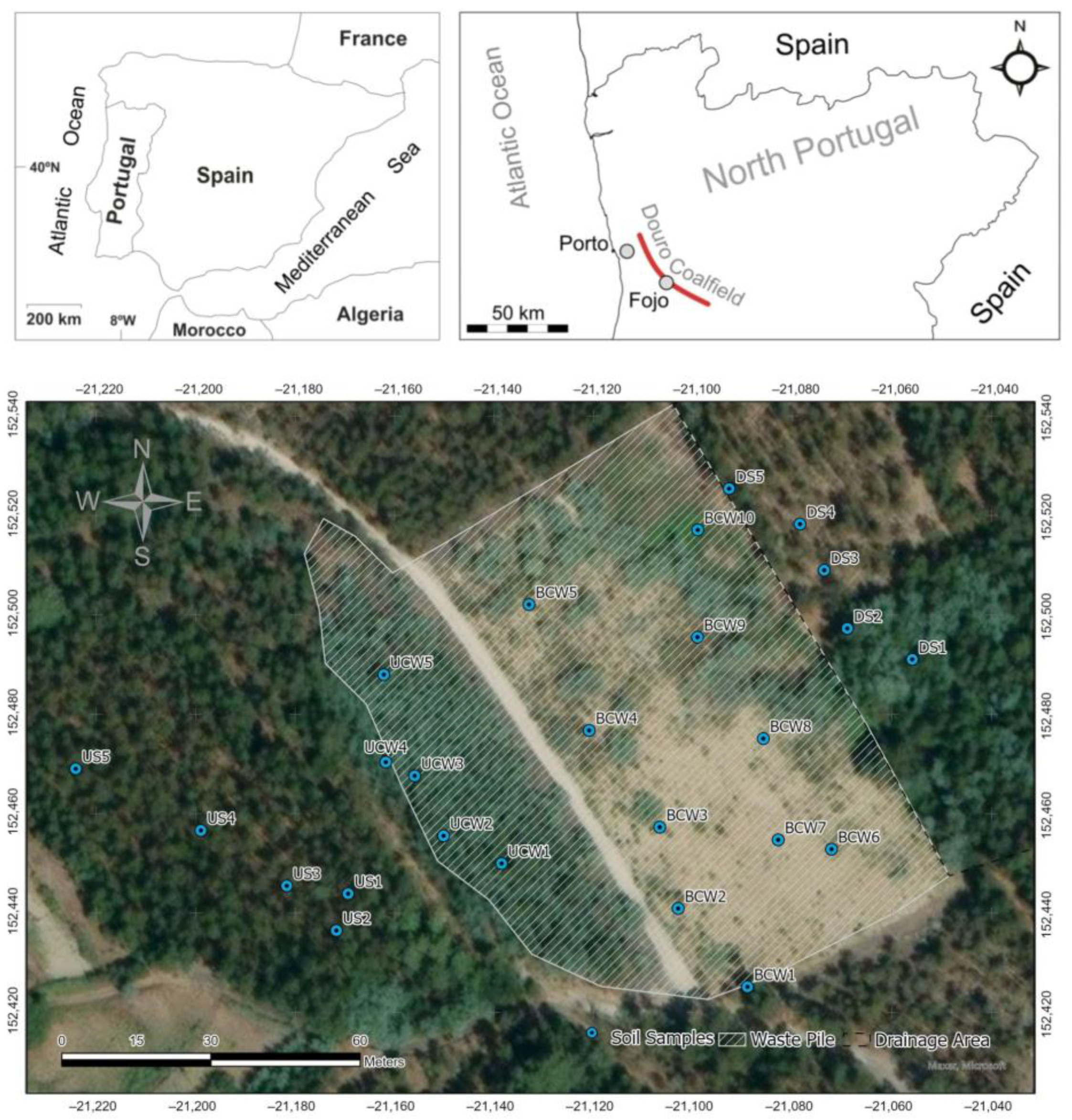
2.1. Soil Sampling
2.2. Chemicals, Solvents and Instruments
2.3. Soil Sample Digestion with Aqua Regia
2.4. Arsenic and Antimony Analysis by HG-AAS
2.5. Arsenic and Antimony Determination
2.6. Sequential Extraction Procedure for As and Sb
3. Results and Discussion
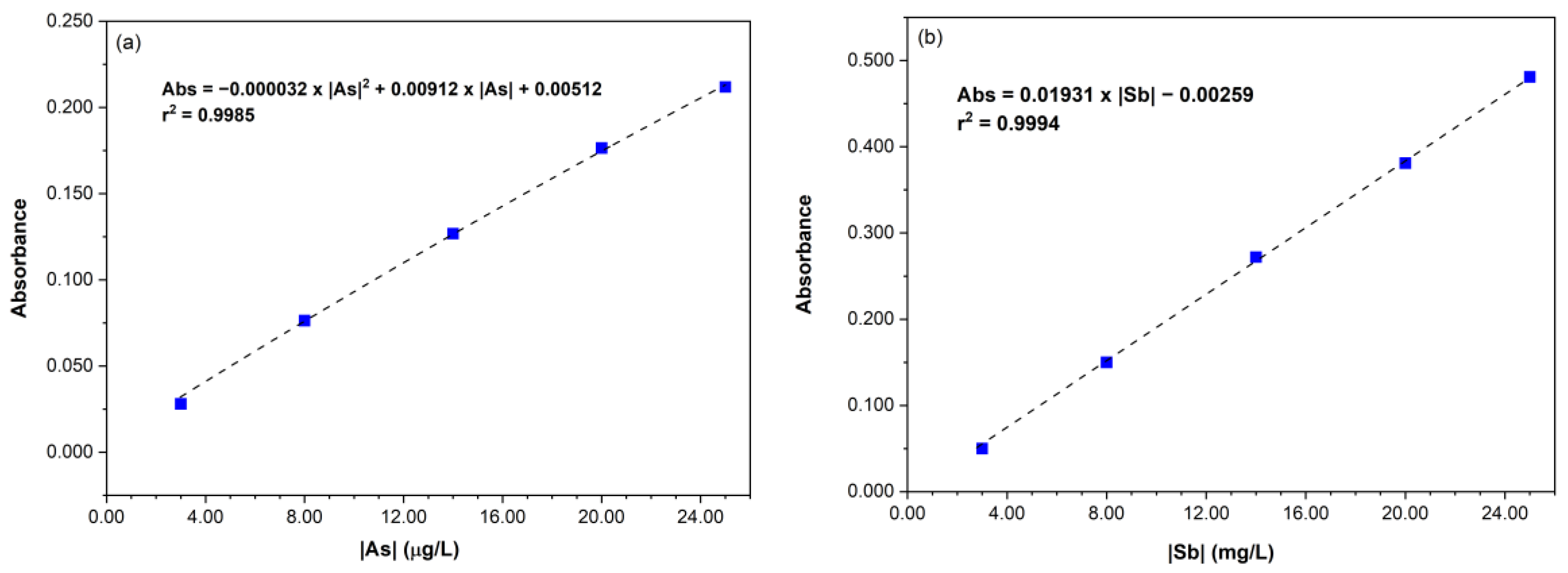
3.1. Arsenic and Antimony Quantification by HG-AAS
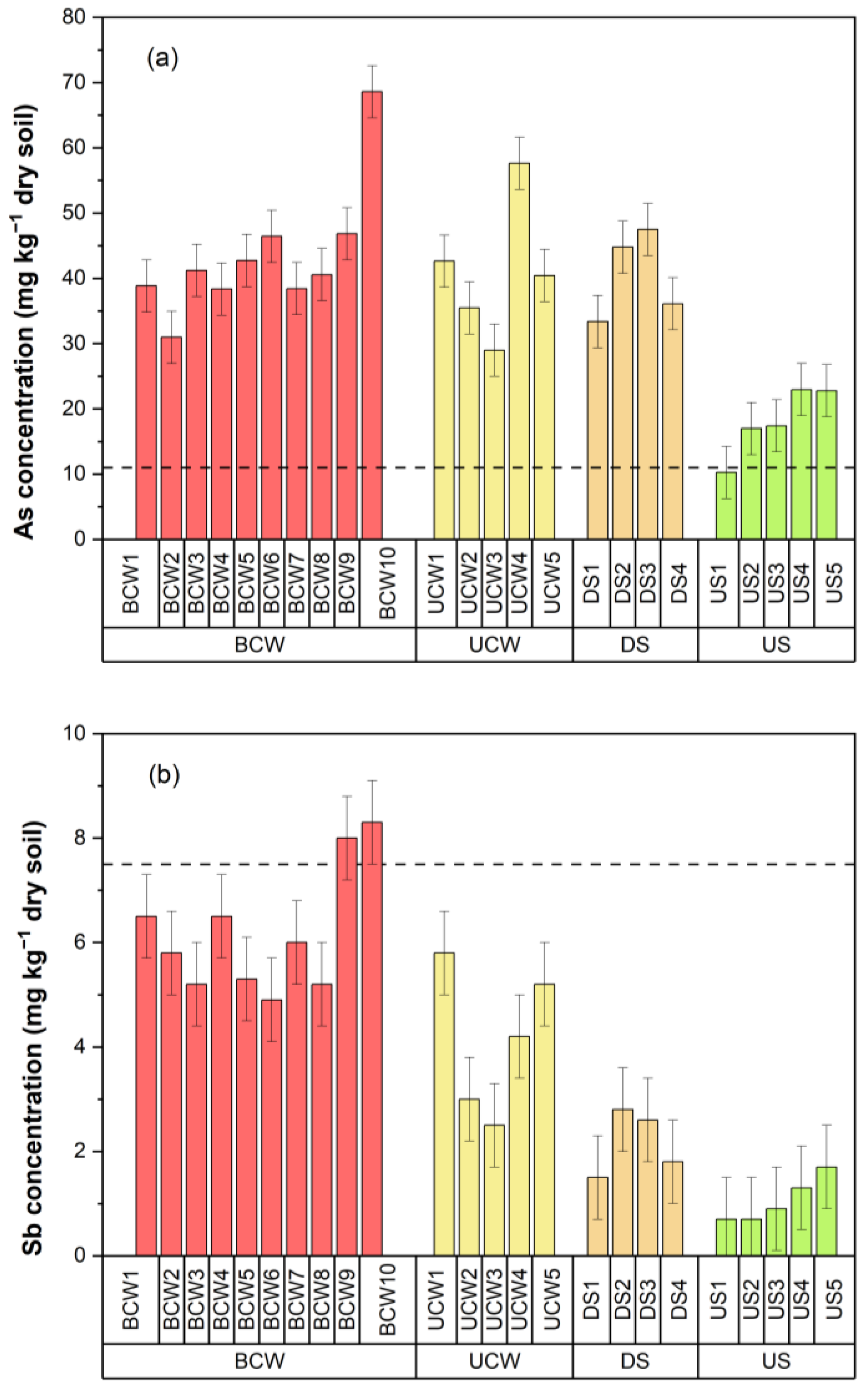
3.2. Aqua Regia Extractable Content of As and Sb in Soil Samples
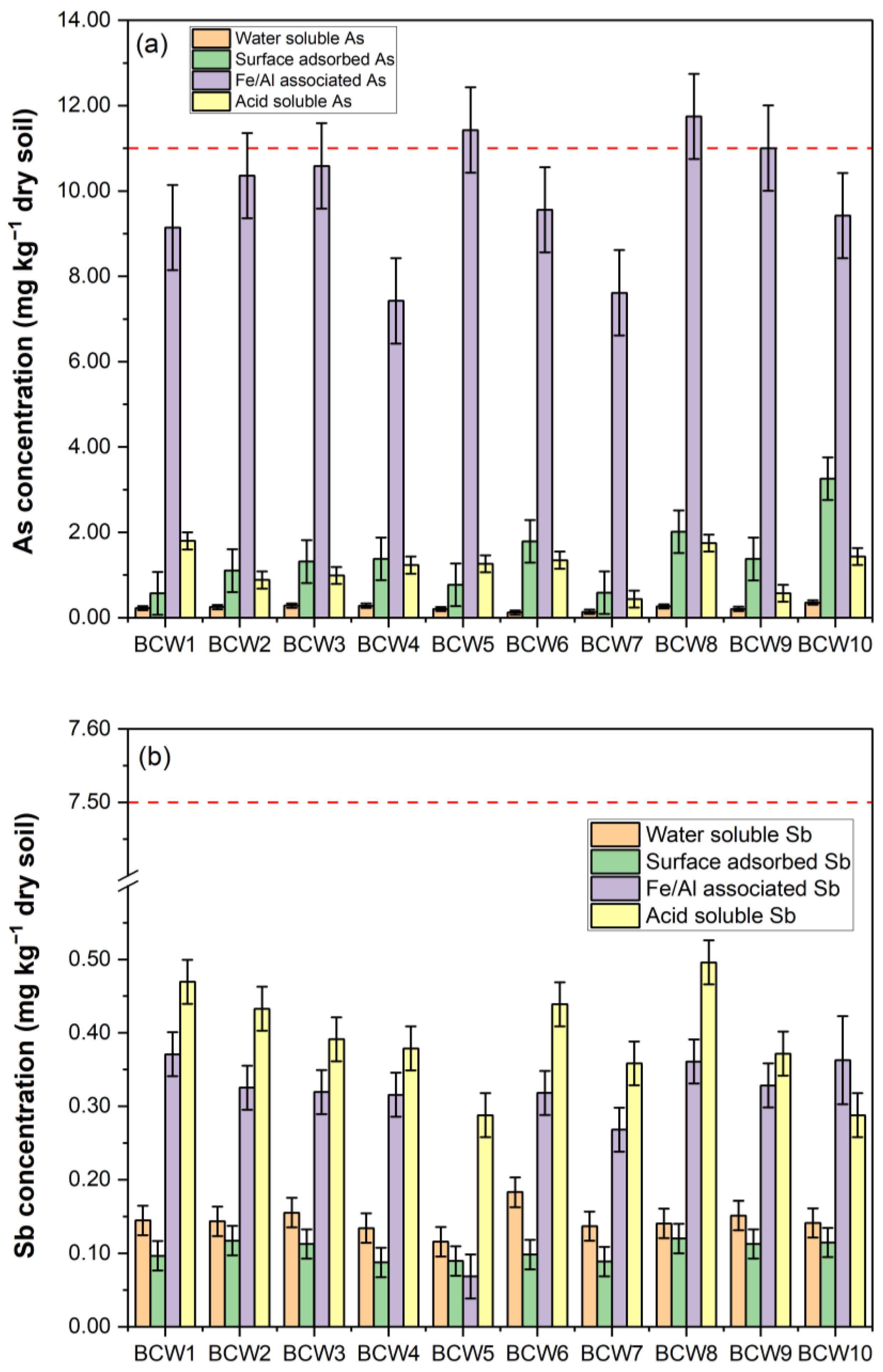
3.3. Fractionating and Mobility of Arsenic and Antimony
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Angon, P.B.; Islam, M.S.; Kc, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Naushad, M.; Lima, E.C.; Zhang, S.; Shaheen, S.M.; Rinklebe, J. Global soil pollution by toxic elements: Current status and future perspectives on the risk assessment and remediation strategies—A review. J. Hazard. Mater. 2021, 417, 126039. [Google Scholar] [CrossRef]
- Naveed, S.; Oladoye, P.O.; Alli, Y.A. Toxic heavy metals: A bibliographic review of risk assessment, toxicity, and phytoremediation technology. Sustain. Chem. Environ. 2023, 2, 100018. [Google Scholar] [CrossRef]
- Monteiro, M.; Santos, P.; Marques, J.E.; Flores, D.; Pereira, C.M.; Ribeiro, J.A.; Azenha, M. Assessment of mobile mercury concentration in soils of an abandoned coalfield waste pile in Douro region: The Fojo waste pile (Portugal) study case. J. Soils Sediments 2024, 24, 2068–2077. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Musilova, J.; Arvay, J.; Vollmannova, A.; Toth, T.; Tomas, J. Environmental Contamination by Heavy Metals in Region with Previous Mining Activity. Bull. Environ. Contam. Toxicol. 2016, 97, 569–575. [Google Scholar] [CrossRef]
- Bacon, J.R.; Davidson, C.M. Is there a future for sequential chemical extraction? Analyst 2008, 133, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.R.M.; Sahuquillo, A.; Lopez Sanchez, J.F. A Review of the Different Methods Applied in Environmental Geochemistry For Single and Sequential Extraction of Trace Elements in Soils and Related Materials. Water Air Soil Pollut. 2008, 189, 291–333. [Google Scholar] [CrossRef]
- Filgueiras, A.V.; Lavilla, I.; Bendicho, C. Chemical sequential extraction for metal partitioning in environmental solid samples. J. Environ. Monit. 2002, 4, 823–857. [Google Scholar] [CrossRef]
- Hass, A.; Fine, P. Sequential Selective Extraction Procedures for the Study of Heavy Metals in Soils, Sediments, and Waste Materials—A Critical Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 365–399. [Google Scholar] [CrossRef]
- Dong, H.; Feng, L.; Qin, Y.; Luo, M. Comparison of different sequential extraction procedures for mercury fractionation in polluted soils. Environ. Sci. Pollut. Res. 2019, 26, 9955–9965. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, M.; Mateu, J.; Rigol, A.; Vidal, M.; López-Sánchez, J.F.; Rauret, G. Use of the modified BCR three-step sequential extraction procedure for the study of trace element dynamics in contaminated soils. Environ. Pollut. 2008, 152, 330–341. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, X.; Guo, Y.; Li, H.; Feng, Y. Simultaneous determination of arsenic, antimony, bismuth and mercury in geological materials by vapor generation-four-channel non-dispersive atomic fluorescence spectrometry. Talanta 2008, 74, 915–921. [Google Scholar] [CrossRef]
- Musil, S.; Matoušek, T. On-line pre-reduction of pentavalent arsenicals by thioglycolic acid for speciation analysis by selective hydride generation–cryotrapping–atomic absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Frentiu, T.; Mihaltan, A.I.; Senila, M.; Darvasi, E.; Ponta, M.; Frentiu, M.; Pintican, B.P. New method for mercury determination in microwave digested soil samples based on cold vapor capacitively coupled plasma microtorch optical emission spectrometry: Comparison with atomic fluorescence spectrometry. Microchem. J. 2013, 110, 545–552. [Google Scholar] [CrossRef]
- Londonio, A.; Fujiwara, F.; Jiménez Rebagliati, R.; Gómez, D.; Smichowski, P. Determination of mercury in size fractionated road dust samples by flow injection-cold vapor-atomic absorption spectrometry. Microchem. J. 2012, 105, 77–82. [Google Scholar] [CrossRef]
- Hu, J.; Li, C.; Zhen, Y.; Chen, H.; He, J.; Hou, X. Current advances of chemical vapor generation in non-tetrahydroborate media for analytical atomic spectrometry. TrAC Trends Anal. Chem. 2022, 155, 116677. [Google Scholar] [CrossRef]
- Costa Ferreira, S.L.; dos Anjos, J.P.; Assis Felix, C.S.; da Silva Junior, M.M.; Palacio, E.; Cerda, V. Speciation analysis of antimony in environmental samples employing atomic fluorescence spectrometry—Review. TrAC Trends Anal. Chem. 2019, 110, 335–343. [Google Scholar] [CrossRef]
- Silva Junior, M.M.; Portugal, L.A.; Serra, A.M.; Ferrer, L.; Cerdà, V.; Ferreira, S.L.C. On line automated system for the determination of Sb(V), Sb(III), thrimethyl antimony(v) and total antimony in soil employing multisyringe flow injection analysis coupled to HG-AFS. Talanta 2017, 165, 502–507. [Google Scholar] [CrossRef]
- Monteiro, M.; Figueiredo, R.; Silva, A.T.; Pereira, C.M.; Azenha, M.; Ribeiro, J.A. Development of a plasmonic sensor based on imprinted nanogels for quantification of bovine serum albumin in bovine milk. Microchem. J. 2025, 209, 112850. [Google Scholar] [CrossRef]
- Espinha Marques, J.; Narayan, A.; Santos, P.; Ribeiro, J.; Antunes, S.C.; Melo, A.; Rocha, F.; Flores, D.; Mansilha, C. Hydropedological Characterization of a Coal Mining Waste Deposition Area Affected by Self-Burning. Hydrology 2024, 11, 62. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, C.; Gong, D.; Deng, Y.; Huang, Y.; Zheng, J.; Xiong, S.; Tang, R.; Wang, Y.; Su, L. A review of the environmental chemical behavior, detection and treatment of antimony. Environ. Technol. Innov. 2021, 24, 102026. [Google Scholar] [CrossRef]
- Herath, I.; Vithanage, M.; Bundschuh, J. Antimony as a global dilemma: Geochemistry, mobility, fate and transport. Environ. Pollut. 2017, 223, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.S.; Pandey, P.K.; Martín-Ramos, P.; Corns, W.T.; Varol, S.; Bhattacharya, P.; Zhu, Y. A review on arsenic in the environment: Contamination, mobility, sources, and exposure. RSC Adv. 2023, 13, 8803–8821. [Google Scholar] [CrossRef]
- Li, J.; Wei, Y.; Zhao, L.; Zhang, J.; Shangguan, Y.; Li, F.; Hou, H. Bioaccessibility of antimony and arsenic in highly polluted soils of the mine area and health risk assessment associated with oral ingestion exposure. Ecotoxicol. Environ. Saf. 2014, 110, 308–315. [Google Scholar] [CrossRef]
- Bagherifam, S.; Brown, T.C.; Fellows, C.M.; Naidu, R. Bioavailability of Arsenic and Antimony in Terrestrial Ecosystems: A Review. Pedosphere 2019, 29, 681–720. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef]
- ISO 12914:2012; Soil Quality—Microwave-Assisted Extraction of the Aqua Regia Soluble Fraction for the Determination of Elements. International Organization for Standardization: Geneva, Switzerland, 2012.
- Element, C. Method 3051A microwave assisted acid digestion of sediments, sludges, soils, and oils. Z. Für Anal. Chem. 2007, 111, 362–366. [Google Scholar]
- Dědina, J. Radical theory of hydride atomization and its significance for trace element analysis. Spectrochim. Acta Part B At. Spectrosc. 2024, 221, 107058. [Google Scholar] [CrossRef]
- Du, X.; Gao, L.; Xun, Y.; Feng, L. Comparison of different sequential extraction procedures to identify and estimate bioavailability of arsenic fractions in soil. J. Soils Sediments 2020, 20, 3656–3668. [Google Scholar] [CrossRef]
- Wan, X.; Dong, H.; Feng, L.; Lin, Z.; Luo, Q. Comparison of three sequential extraction procedures for arsenic fractionation in highly polluted sites. Chemosphere 2017, 178, 402–410. [Google Scholar] [CrossRef]
- Shiowatana, J.; McLaren, R.G.; Chanmekha, N.; Samphao, A. Fractionation of Arsenic in Soil by a Continuous-Flow Sequential Extraction Method. J. Environ. Qual. 2001, 30, 1940–1949. [Google Scholar] [CrossRef]
- Mitsunobu, S.; Harada, T.; Takahashi, Y. Comparison of Antimony Behavior with that of Arsenic under Various Soil Redox Conditions. Environ. Sci. Technol. 2006, 40, 7270–7276. [Google Scholar] [CrossRef]
- Okkenhaug, G.; Zhu, Y.-G.; He, J.; Li, X.; Luo, L.; Mulder, J. Antimony (Sb) and Arsenic (As) in Sb Mining Impacted Paddy Soil from Xikuangshan, China: Differences in Mechanisms Controlling Soil Sequestration and Uptake in Rice. Environ. Sci. Technol. 2012, 46, 3155–3162. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Melaku, S.; Dams, R.; Moens, L. Determination of trace elements in agricultural soil samples by inductively coupled plasma-mass spectrometry: Microwave acid digestion versus aqua regia extraction. Anal. Chim. Acta 2005, 543, 117–123. [Google Scholar] [CrossRef]
- Santoro, A.; Held, A.; Linsinger, T.P.J.; Perez, A.; Ricci, M. Comparison of total and aqua regia extractability of heavy metals in sewage sludge: The case study of a certified reference material. TrAC Trends Anal. Chem. 2017, 89, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.; Suárez-Ruiz, I.; Flores, D. Coal related fires in Portugal: New occurrences and new insights on the characterization of thermally affected and non-affected coal waste piles. Int. J. Coal Geol. 2022, 252, 103941. [Google Scholar] [CrossRef]
- Costa, M.; Moura, H.; Pinto de Jesus, A.; Suárez-Ruiz, I.; Flores, D. Effects of Magmatic Fluids in Coals of São Pedro da Cova Coalfield, Douro Carboniferous Basin, Portugal: Insights from Inorganic Geochemistry. Minerals 2022, 12, 275. [Google Scholar] [CrossRef]
- Swain, C.K. Environmental pollution indices: A review on concentration of heavy metals in air, water, and soil near industrialization and urbanisation. Discov. Environ. 2024, 2, 5. [Google Scholar] [CrossRef]
- Finkelman, R.B. Potential health impacts of burning coal beds and waste banks. Int. J. Coal Geol. 2004, 59, 19–24. [Google Scholar] [CrossRef]
- Querol, X.; Fernández-Turiel, J.; López-Soler, A. Trace elements in coal and their behaviour during combustion in a large power station. Fuel 1995, 74, 331–343. [Google Scholar] [CrossRef]
- Liu, H.; Pan, W.-P.; Wang, C.; Zhang, Y. Volatilization of Arsenic During Coal Combustion Based on Isothermal Thermogravimetric Analysis at 600–1500 °C. Energy Fuels 2016, 30, 6790–6798. [Google Scholar] [CrossRef]
- Xu, F.; Chu, M.; Hao, C.; Zhou, L.; Sun, X.; Gu, Z. Volatilization characteristics and relationship of arsenic and sulfur during coal pyrolysis. Fuel 2022, 315, 123223. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Silva, A.F.; Girault, H.H.; Pereira, C.M. Electroanalytical applications of ITIES—A review. Talanta 2024, 280, 126729. [Google Scholar] [CrossRef]
- Joana, T.; José, A.R.; Pedro, A.S.J.; Nuno, A.S. Probing molecular affinity with optical tweezers. In Proceedings of the SPIE Photonics Europe, Strasbourg, France, 7–11 April 2024; p. 1299105. [Google Scholar]
- Pedro, J.; Joana, T.; Vicente, R.; José, R.; Nuno, S. Automation of optical tweezers: An enabler for single cell analysis and diagnostic. In Proceedings of the SPIE Photonics Europe, Strasbourg, France, 7–11 April 2024; p. 130080E. [Google Scholar]
- Teixeira, J.; Ribeiro, J.; Silva, N.; Jorge, P. Automated Optical Tweezers for Enhanced Bioparticle Analysis via Combined Scattering and Raman Spectroscopy. In Proceedings of the 2024 IEEE Sensors Applications Symposium (SAS), Naples, Italy, 23–25 July 2024; pp. 1–6. [Google Scholar]
- Gonçalves, J.; Araújo, A.; Pedron, T.; Santos, R.; Bouguerra, S.; Ribeiro, J.A.; Pereira, R.; Pereira, C.M.; Azenha, M. Discarded substrates from soilless hydroponic horticulture as potential amendments for metal-contaminated soils. Chemosphere 2024, 364, 143127. [Google Scholar] [CrossRef]

| AAS and VP100 Operating Conditions | |
|---|---|
| Slit width | 0.5 nm |
| Burner height | 15 mm |
| Hollow lamp wavelength (As) | 217.6 nm |
| Hollow lamp wavelength (Sb) | 193.7 nm |
| Lamp current/voltage | 75%/6 V |
| Signal measurement | Transient Height |
| Signal type | Background correction |
| Total measurement time | 100 s |
| Sample aspiration time | 25 s |
| Carrier gas flow | 100 mL·min−1 |
| Pump speed | 40 rpm |
| Sample uptake rate | 7.5 mL·min−1 |
| Reducing agent uptake rate | 1.6 mL·min−1 |
| Acid uptake rate | 0.7 mL·min−1 |
| Step | Defined Fraction | Extracting Solution | Experimental Conditions |
|---|---|---|---|
| 1 | Water soluble | 30 mL of ultrapure water | Shake for 16 h at 25 °C |
| 2 | Surface adsorbed | 30 mL of NaHCO3 0.5 M (pH = 9) | Shake for 16 h at 25 °C |
| 3 | Associated with Fe/Al | 30 mL of NaOH 0.1 M (pH = 13) | Shake for 16 h at 25 °C |
| 4 | Acid extractable (a) | 30 mL of HCl 1 M | Shake for 16 h at 25 °C |
| Arsenic | Antimony | |||
|---|---|---|---|---|
| Analytical Parameter | Statistical Parameter | Value (µg mL−1) | Statistical Parameter | Value (µg mL−1) |
| LOD | Sy/x = 0.003 | 0.50 | Sy/x = 0.003 | 0.68 |
| LOQ | 2.8 | 2.1 | ||
| Sensitivity | Abs (8 µg L−1) = 0.076 | 0.50 | Abs (8 µg L−1) = 0.076 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, M.; Santos, P.; Espinha Marques, J.; Flores, D.; Azenha, M.; Ribeiro, J.A. Assessment of Potential Environmental Risks Posed by Soils of a Deactivated Coal Mining Area in Northern Portugal—Impact of Arsenic and Antimony. Pollutants 2025, 5, 15. https://doi.org/10.3390/pollutants5020015
Monteiro M, Santos P, Espinha Marques J, Flores D, Azenha M, Ribeiro JA. Assessment of Potential Environmental Risks Posed by Soils of a Deactivated Coal Mining Area in Northern Portugal—Impact of Arsenic and Antimony. Pollutants. 2025; 5(2):15. https://doi.org/10.3390/pollutants5020015
Chicago/Turabian StyleMonteiro, Marcus, Patrícia Santos, Jorge Espinha Marques, Deolinda Flores, Manuel Azenha, and José A. Ribeiro. 2025. "Assessment of Potential Environmental Risks Posed by Soils of a Deactivated Coal Mining Area in Northern Portugal—Impact of Arsenic and Antimony" Pollutants 5, no. 2: 15. https://doi.org/10.3390/pollutants5020015
APA StyleMonteiro, M., Santos, P., Espinha Marques, J., Flores, D., Azenha, M., & Ribeiro, J. A. (2025). Assessment of Potential Environmental Risks Posed by Soils of a Deactivated Coal Mining Area in Northern Portugal—Impact of Arsenic and Antimony. Pollutants, 5(2), 15. https://doi.org/10.3390/pollutants5020015









