Abstract
In this paper, we analyse the effectiveness of microbubble technology in inactivating/reducing gram-negative, gram-positive, and radiation-resistant bacteria, including Escherichia coli, Bacillus subtilis, and Deinococcus radiodurans, respectively, in model water. Key water quality parameters such as dissolved oxygen, conductivity, pH, and total dissolved solids are recorded and presented to demonstrate their range in the current investigation. The study results indicate a reduction of 95% in E. coli, 100% in D. radiodurans, and 45% in B. subtilis following microbubble treatment. These findings suggest that ambient air microbubbles, generated using a low-cost, reagent-free, and eco-friendly venturi-type microbubble generator, represent a promising technique for reducing bacterial loads in water.
1. Introduction
A major threat to the world’s water supplies today is the massive generation of wastewater resulting from rapid industrialisation and the population. Consequently, wastewater reclamation and reuse have become essential to meet human needs in the face of limited freshwater resources [1]. However, a key concern with reclaimed water is that it may contain various types of resistant microorganisms and persistent organic pollutants. Bacterial contamination and the resulting infections are widely recognised as serious threats to human health, making the prevention of waterborne diseases critical for ensuring water safety [2].
Bacteria can be classified as gram-positive or gram-negative based on their morphology and the differential staining properties of the organism. This classification is primarily due to differences in their cell wall structures, with gram-positive bacteria having a thicker peptidoglycan layer. Additionally, bacteria can be categorised by shape, such as cocci (spherical or ball-shaped) and bacilli (rod-shaped). These classifications are important in determining effective treatment approaches, as different bacteria may respond differently to various disinfection methods.
Disinfectants play a crucial role in hygiene management and have long been used as a cost-effective means of controlling bacterial infections. In clinical settings, strong disinfectants such as chlorine and ozone are routinely employed. Although chlorine-based disinfectants are commercially affordable and effective, their interaction with organic compounds can lead to the formation of trihalomethanes—known carcinogens [3]. As a result, chlorination and ozonation are not considered environmentally friendly. Furthermore, some microorganisms form bacterial colonies and spores that cluster together into biofilms or dense spherical structures [4]. Complete cell disintegration of such biofilms is not seen because chemical treatment of such a cluster only destroys the top layer, leaving the underneath layer intact. The discovery of an alternate disinfectant is very critical.
To address hygiene challenges in developing nations, numerous novel disinfection approaches, such as solar disinfection [5] and Mixed Oxidant Gases Systems, are being explored. However, these methods face limitations due to inadequate light penetration and reduced effectiveness against radiation-resistant organisms or those with photoreactivation repair mechanisms. Hence, there is a critical need for the development of newer, more efficient, and cost-effective water disinfection technologies that can effectively inactivate or eliminate microorganisms by disrupting their cellular structures or interfering with their metabolic and biosynthetic processes [6].
Many researchers have found that hydrodynamic cavitation could be another potentially useful non-reagent approach. Many forms of hydrodynamic cavity generators have been used in the past to inactivate bacteria, viruses, and yeasts [7]. Hydrodynamic cavity generators can be categorised as either non-rotational (based on the constriction effect) or rotating (based on the shear effect) depending on the cavitation-generating mechanism [8]. Due to their high commercial viability, straightforward construction, and simplicity of use, non-rotating hydrodynamic cavity generators such as orifices or venturis are extensively explored in a variety of applications like pollutant degradation, food processing, and disinfection [9]. During hydrodynamic-cavitation disinfection, the microbes are subjected to several cavitation effects and, as a result, undergo damage or disintegration, making them unable to divide and cause diseases. Despite the high electricity consumption, hydrodynamic cavitation is simple and follows through quick disinfection without the need for reagents. The apparatus is affordable and could be utilised in houses or small community water treatment plants. When compared to the most common disinfection method, i.e., chlorination disinfection, microbubble-driven oxidation does not produce any harmful by-products and is one of the most promising techniques in water disinfection.
Microbubbles (MBs) are small bubbles with diameters ranging from 10 to 100 µm. The shared properties of these MBs that distinguish them from typical macrobubbles are their extended residence time span, smaller buoyancy, self-pressuring effect, and substantial gas–liquid interfacial area. Because of its potential to generate reactive free radicals, microbubble technology has received a lot of interest in water treatment over the last ten years. Unlike the conventional aeration or disinfection systems, which employ either reactive oxidative species (such as ozone or UV irradiation) or oxidative stress induced by hypochlorous acid, such as in chlorination, microbubbles offer distinct advantages of coupling physical and chemical mechanisms of disinfection. They generate localised high temperatures and pressure on collapse or coalescence, which leads to the formation of reactive oxygen species such as hydroxyl radicals (OH.) or superoxide anions (02.-). These reactive oxygen species can lead to effective microbial inactivation by damaging the microbial cell walls and disrupting the membrane structure. Beyond their chemical action, physical effects caused by their buoyancy-induced rise and subsequent collapse also create shear forces within the fluid facilitating biofilm detachment and the weakening of the membrane structure. Most importantly, this approach works without the need for any chemical agents, significantly reducing the formation of harmful byproducts and thereby contributing to environmental sustainability [10].
Ozone microbubbles have been shown to successfully inactivate a wide range of microorganisms via cell lysis and nucleic acid degradation [11,12]. The low utilisation efficiency of conventional ozonation was also overcome with the use of ozone microbubbles. The effect of inlet ozone concentration on the disinfection efficiency was comprehensively investigated by Zhang et al. [13]. The log reduction in B. subtilis by microbubble ozonation increased from log 0.5 at 40 mg/L to log 5 at 140 mg/L ozone concentration, after 3 min of operation. This is supported by the fact that the size of bubbles decreases in the greater ozone inlet, resulting in a higher volumetric mass transfer coefficient and, as a result, increased utilisation efficiency Zhang et al. [13]. Hydrodynamic cavitation was found to be particularly effective in limiting bacteria’s ability to divide in experiments conducted by Mezule et al. [14] E. coli cells were stopped from dividing after 3 min of exposure at 490 W/L [14,15] studied the capacity of CO2 microbubbles to inactivate Escherichia coli (thermotolerant coliform) suspended in a saline solution at pressures less than 2 MPa and temperatures less than 40° C. After 60 min of microbubble-aided CO2 treatment at 40 °C and 2.0 MPa, the E. coli population was reduced by 6 logs.
Jain et al. [16] have used two different cavitation reactors, orifice, and vortex diode for evaluating the disinfection of both gram-positive (Staphylococcus aureus) and gram-negative (Escherichia coli) bacteria. The reactor geometry was found to have a significant effect on the process of disinfection, and a rotating flow vortex cavitating device was found to be more effective than the linear flow orifice-type cavitating device for the destruction of pathogenic bacteria. After 1 h of cavitation at a pressure drop of only 0.5 bar, E. coli was completely eradicated (99%). However, the removal of S. aureus was shown to be less effective than the removal of E. coli using a vortex diode, with only 60% disinfection accomplished under identical circumstances. The removal efficacy of S. aureus can be increased to 98% by increasing the pressure drop [16].
The purpose of the present study is to evaluate the feasibility and effectiveness of using microbubble technology in inactivating/reducing gram-negative, gram-positive, and radiation-resistant bacteria. Escherichia coli, Bacillus subtilis, and Deinococcus radiodurans were used as model organisms in this investigation. To achieve the objectives of this study, a microbubble aeration system developed by Basso et al. [17] and Hamad et al. [18] was employed to generate the microbubbles required for the experiments.
2. Material and Methods
Liquid cultures of Escherichia coli K12 were obtained from Mary Elizabeth Pownall at the University of York, Deinococcus radiodurans R1 was as reported in Davis et al. [19], and Bacillus subtilis W23 was from Anthony Wilkinson at the University of York. E. coli was grown on Luria–Bertani broth at 37 °C and D. radiodurans and B. subtilis were both grown on YPD media at 30 °C, respectively. A known concentration of each of the bacterial cultures was added to 30 L of deionised water in a glass tank, individually, to obtain a total bacterial concentration of approximately 103 colony-forming units (CFU)/mL of E. coli, D. radiodurans, and B. subtilis, respectively. Nutrient agar and nutrient broth (Oxoid) were purchased from Thermofischer scientific, Horsham, UK. General purpose grade sodium chloride (NaCl) was purchased from Thermofischer scientific, UK.
The three bacterial species selected—Escherichia coli, Bacillus subtilis, and Deinococcus radiodurans—represent phylogenetically diverse and functionally distinct model organisms. E. coli (gram-negative, Proteobacteria) is a widely used model for enteric pathogens and a frequent contaminant in healthcare settings, known to persist on hospital surfaces and medical devices, contributing to hospital-acquired infections [20,21]. B. subtilis (gram-positive, Firmicutes) serves as a model spore-forming bacterium with relevance to both biomedical environments—such as wound infections and polymicrobial biofilms—and industrial sectors, including food production, where it contaminates stainless steel surfaces, and pharmaceutical cleanrooms, where it resists conventional sterilisation [21,22,23] D. radiodurans (Deinococcus–Thermus clade) is an extremophile with remarkable resistance to ionising radiation and desiccation; it is not only a model for stress biology but also a known persistent contaminant in nuclear waste treatment systems, paper production facilities, and precision industrial settings such as aerospace and semiconductor manufacturing [24]. These organisms also possess fundamentally distinct cell envelope architectures—E. coli has a thin peptidoglycan layer and outer membrane, B. subtilis features a thick multilayered peptidoglycan wall, and D. radiodurans displays a complex, atypical envelope with distinct layers—each of which is known to confer differential susceptibility to lytic and oxidative stresses [25]. Accordingly, we hypothesised from the outset that their disinfection responses to microbubble treatment would diverge.
2.1. Experiment Setup
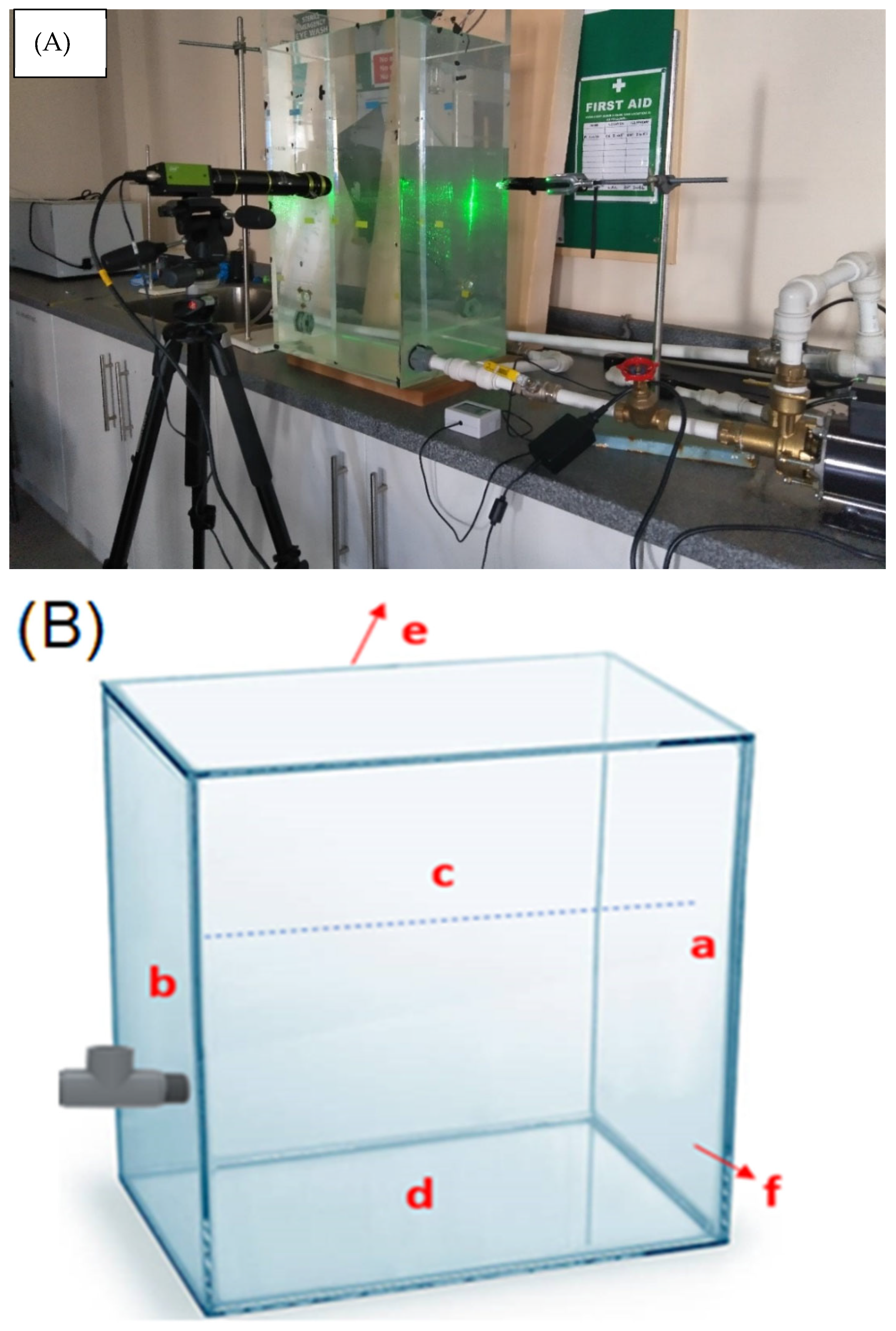
The experiment was performed in a transparent 80 L water tank [17] and Hamad et al. [18]. The water storage tank, pump, cavitating device, i.e., venturi microbubble generator, control mechanism for parameters such as air and water flow rate, and high-speed camera are vital components of the setup. Figure 1 shows an image of the experimental arrangement. A venturi-type MB generator is connected to a glass tank, which is connected to a water pump with a water flow rate of 7.75 LPM. The air was fed into the system at a flow rate of 0.1 L/min. The controlled air was then mixed with circulating water inside the microbubble generator (venturi meter) to form air microbubbles and subsequently returned to the water in the tank. A high-definition camera (Stemmer Jai camera-AP-3200 T-USB, JAI Inc., San Jose, CA, USA) was used to determine the bubble size.
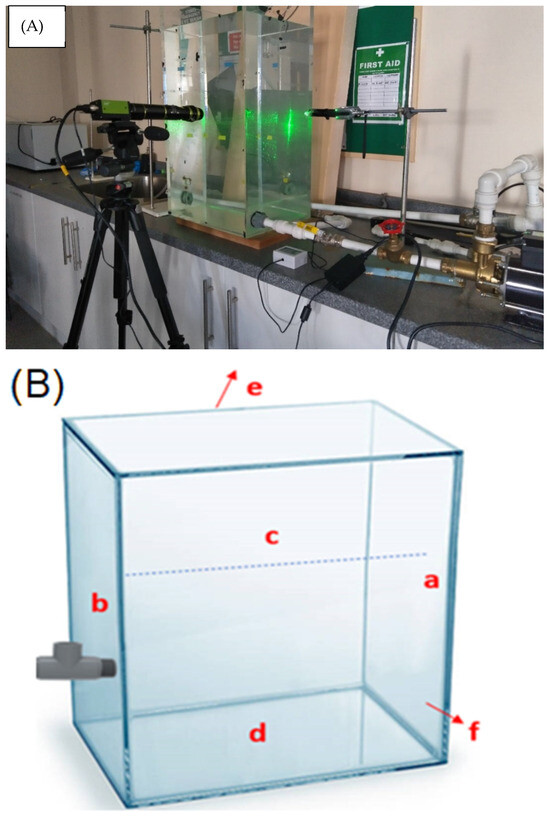
Figure 1.
(A) Photograph of the experimental setup [18]. (B) Sampling points (marked as a—opposite to the main wall of the tank, b—wall fitted with venturi (main wall), c—surface, d—bottom, e—back wall, and f—front wall) from the glass tank.
The thoroughly cleaned glass tank was filled with 30 L of deionised water, and the desired concentration of bacteria was added to the water tank and mixed well. Deionised water serves as a baseline reference point, enabling the establishment of the fundamental properties and behaviours of microbubbles in a well-defined environment before exploring more complex water matrices as found in natural surface water. Following the setup of the desired flow through the venturi meter, the tank water was bubbled with air microbubbles and samples were collected at different intervals of time from different points in the tank. Water was sampled from the four corners and the surface and bottom of the tank (5 mL per spot). Intermittent microbubbling types of bubbling techniques were employed. The intermittent bubbling experiment is the microbubbling of water for a certain time and leaving it undisturbed alternatively. Samples (5 mL) were withdrawn at regular intervals. The temperature in degrees Celsius (T°), dissolved oxygen (DO), pH, conductivity, and total dissolved solids (TDS) were all measured using a multi-parameter water quality meter (Fisher brand™ Traceable™ Portable Dissolved Oxygen Meter) prior to the treatment and after every hour during the run of the microbubble generator. Colony-forming units (CFUs) were determined for each sample, as stated in the following section. To represent variability and reproducibility, experiments with all three bacteria were repeated three times, and the mean value was considered; in addition, reproducibility experiments were conducted using three different initial bacterial concentrations (102, 103, and 104 CFU/mL) to investigate the robustness of the disinfection effectiveness under variable bacterial lots. The microbubble-based disinfection system showed consistent bacterial inactivation across all concentrations, demonstrating robust repeatability. The reproducibility of the experiments was examined and found to be satisfactory (within 4% for most of the experiments).
To ensure that the bacterial counts reflected the effects of the microbubble treatment rather than gravitational sedimentation or the bacterial movement due to hydrodynamic forces, the sampling procedures were implemented as follows: (i) the samples were collected from six different points in the tank—including the sides, surface, and bottom—to assess spatial variations; and (ii) the data were collected at different times to evaluate temporal variation.
2.2. Disinfection Activity
The spread plate count method was used to count the number of viable bacteria present in the model contaminated water. The samples were serially diluted 10-fold with a sterile saline solution (NaCl 0.85%). Each diluted solution was put on a 90 mm sterile nutritional agar plate using 0.1 mL and spread using a sterilised glass rod to avoid contamination. After 48 h of incubation at 30 °C, the colonies of surviving bacteria on agar plates were counted and presented as colony-forming units per millilitre (CFU/mL).
The following equation was used to calculate the colony-forming unit:
After the microbubble treatment, disinfection efficiency or log reduction is calculated using the equation given below:
where a = initial bacterial count CFU/mL and b = final bacterial count after treatment.
The percentage of bacterial reduction is calculated based on the formula below.
The % reduction in the concentration of different bacteria before and after air microbubble treatment is then evaluated.
3. Result and Discussion
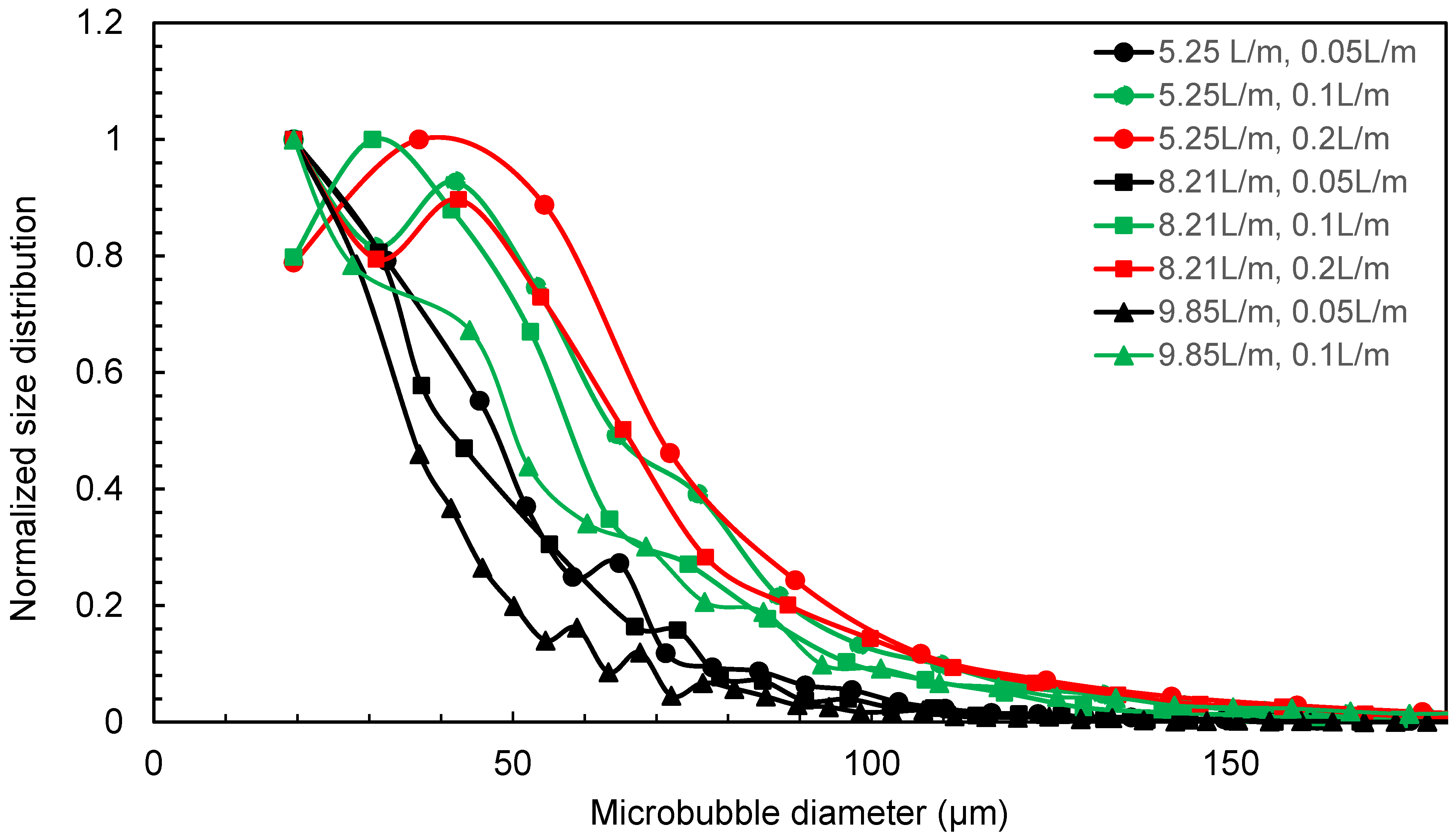
Venturi-type microbubble generators were chosen as they are energy-efficient compared to other methods [17,18]. The pressure differential created by the venturi effect allows for the injection of the controlled volume of air/gas into the liquid without the need for additional energy-intensive equipment. The venturi-type microbubble generator produces an average microbubble size in the range of 50–70 μm size for the water flow rate in the range of 5.25–9.85 L/min, as given in Figure 2 [18], and to confirm that the air bubble sizes are within the range for the microbubble size, the water flow rate of 7.75 L/min and air flow rate of 0.1 L/min was used in the tests.
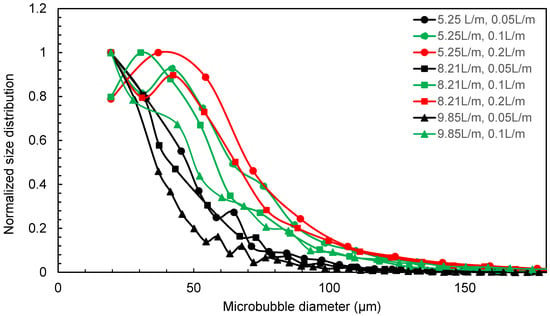
Figure 2.
Microbubble diameter distribution from venturi microbubble generator for different water and air flow rates [18].
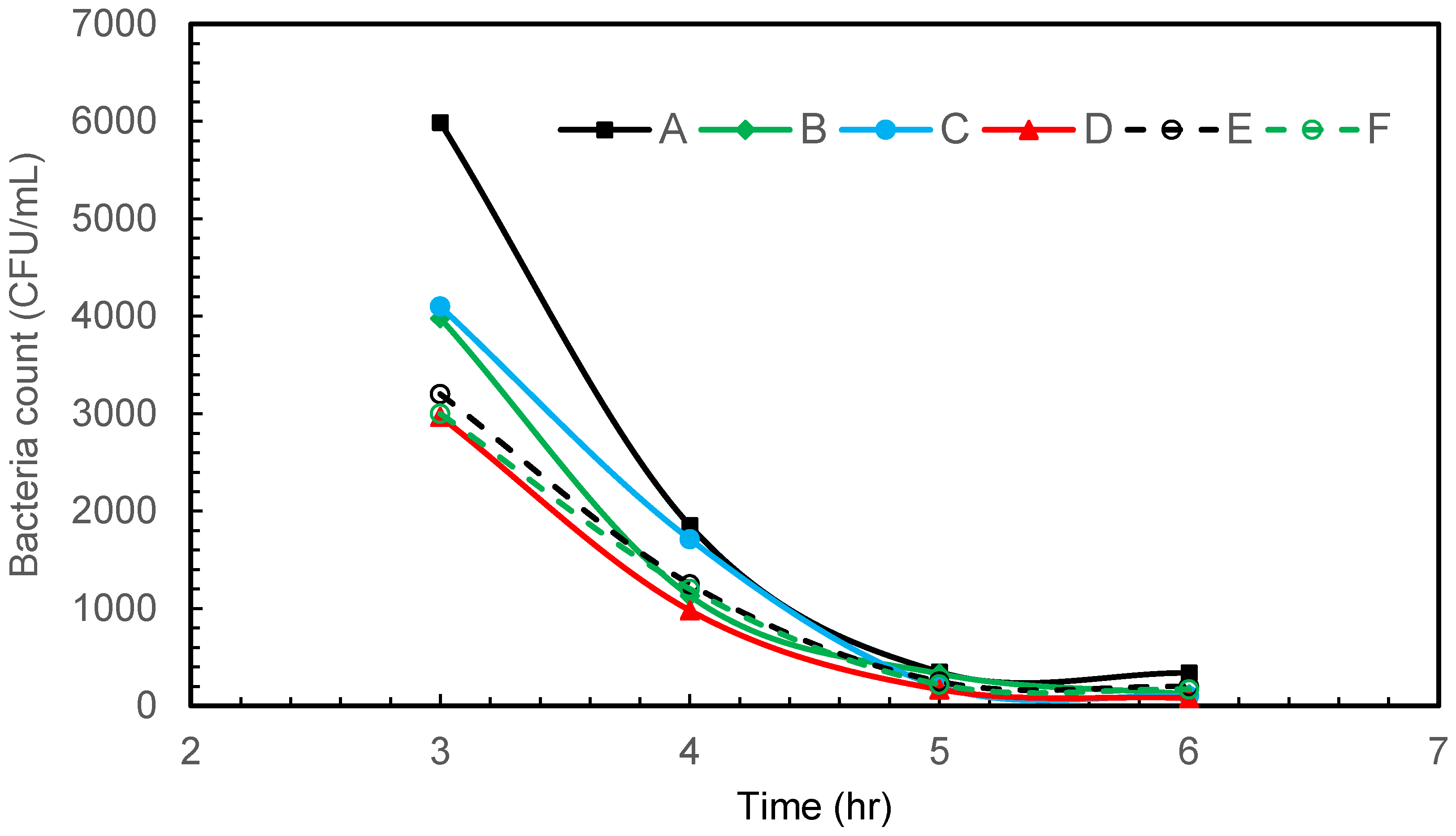
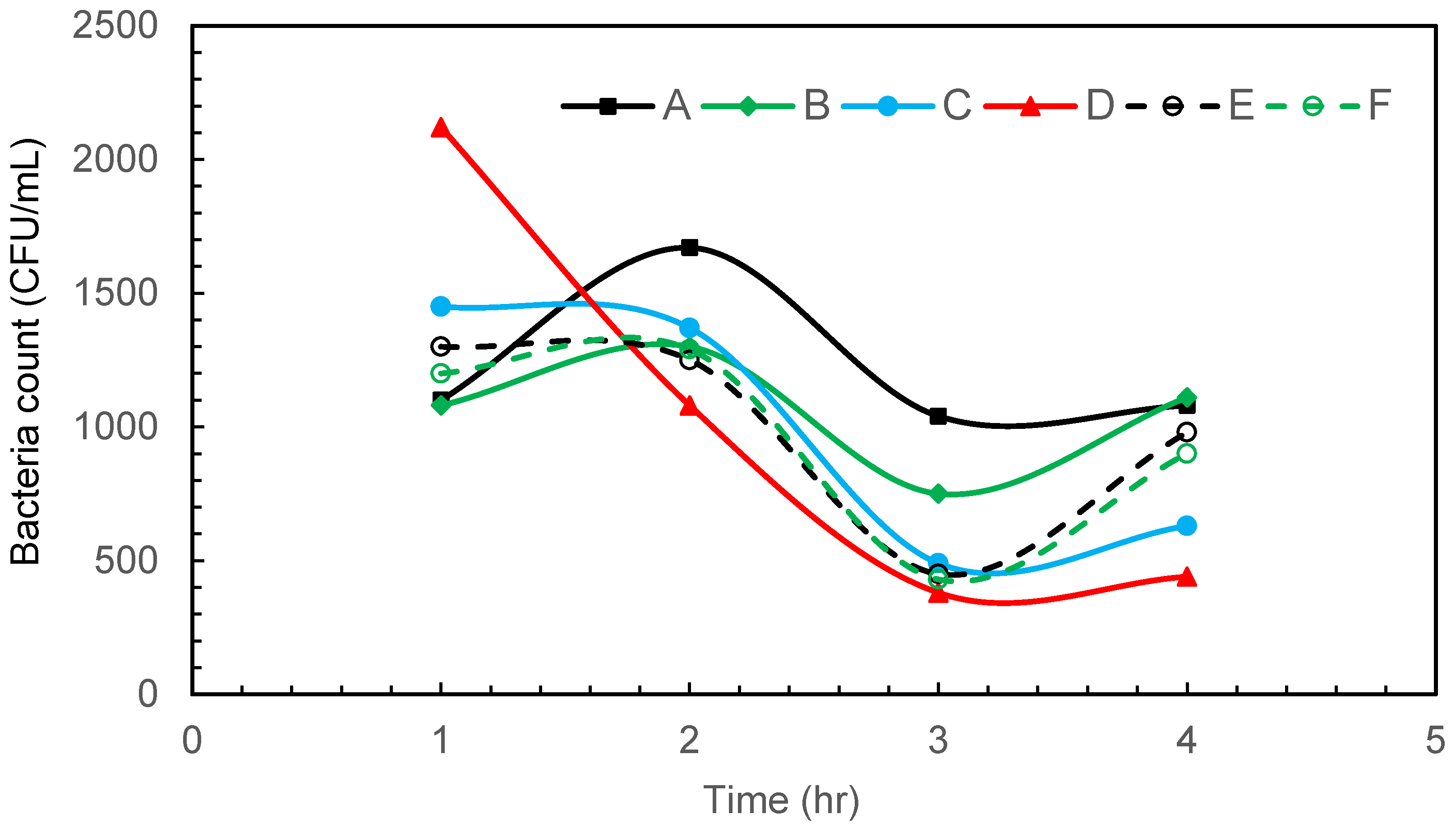
Figure 3 depicts the results of gram-negative bacteria E. coli disinfection at various sampling points in the glass tank using air microbubbles produced from a venturi-based microbubble generator. The flow direction is influenced by the overall design of the tank and the positioning of the microbubble generator. Turbulence may be present, especially in the vicinity of the microbubble injection point, as the microbubbles mix with the water. The tank itself plays a role in determining the flow pattern. The dimensions, shape, and features of the tank can influence how water circulates and how microbubbles are distributed. At the start of the bubbling, most bacteria are accumulating on the wall opposite the venturi (Figure 3). This could be attributed to the fluid flow pattern from the venturi meter, which pushes most of the bacteria to the opposite wall in the beginning.
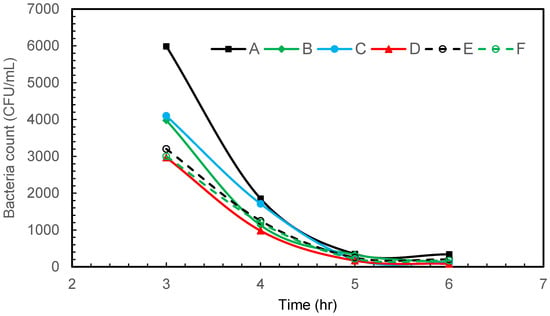
Figure 3.
Intermittent microbubbling of E. coli contaminated water with 6 sampling points in tank.
Within 6 h of intermittent microbubbling and cavitation, E. coli was effectively eliminated and a 95.65% reduction in bacterial count was achieved by the end of the treatment period, which is associated with a log reduction of 1.36. As reported by several other researchers, it is believed that disinfection or a reduction in bacteria occurs due to a synergistic combination of shear, oxidation, and heating effects. As a venturi-type microbubble generator is used in this study, the shrinkage rate of the collapsing bubbles is extremely slow and will not increase the temperature of the water. Due to pyrolytic decomposition that takes place within the collapsing bubbles, the OH radical and shock waves can be generated at the gas–liquid interface, which in turn is responsible for the oxidative cell damage of microbes [26,27]. In addition to this, the physical rupture of the cell is known to take place due to shock/waves generated by bubble collapse. The generation of highly reactive free radicals and turbulence associated with the collapsing MBs provides great potential for water disinfection.
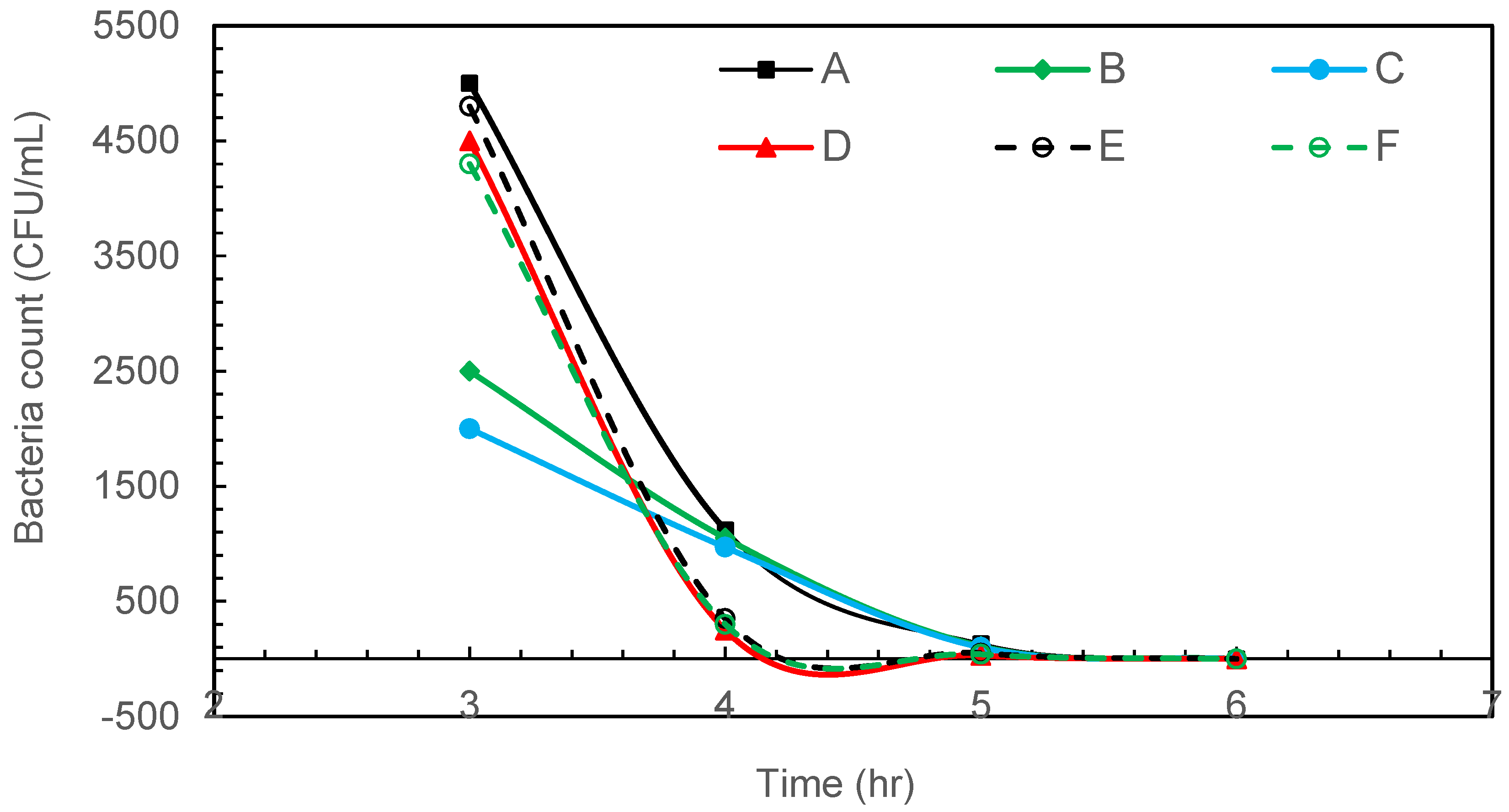
Figure 4 shows the result of radiation-resistant D. radiodurans disinfection with intermittent microbubbling. The complete removal of radiation-resistant D. radiodurans was observed after 5 h of intermittent air microbubble treatment.
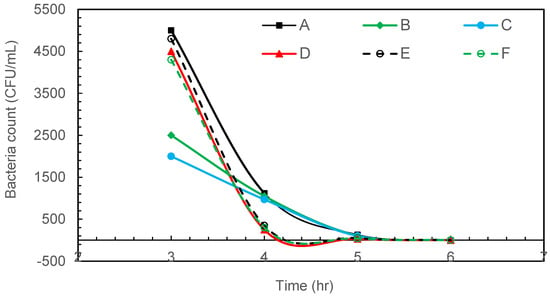
Figure 4.
Intermittent microbubbling of D. radiodurans contaminated water with 6 sampling points in tank.
While D. radiodurans is renowned for its exceptional resistance to ionising radiation and desiccation, this robustness primarily stems from efficient DNA repair systems and oxidative stress responses rather than from a highly protective cell wall. Its cell envelope, though structurally unusual, lacks the thick protective peptidoglycan layers seen in gram-positive bacteria [25]. Therefore, it is not surprising that this species was fully inactivated by microbubble treatment, which exerts its effect through physical rupture and oxidative damage at the cell surface. The observed 100% reduction is thus consistent with known vulnerabilities in the cell envelope rather than unexpected experimental error.
Figure 5 shows the result of gram-positive B. subtilis disinfection with intermittent microbubbling at different sampling points in the tank. A different phenomenon was observed, wherein a higher number of B. subtilis bacteria accumulated near the surface in contrast to the behaviour of the earlier two types of bacteria, suggesting a potential tendency for surface adhesion and biofilm formation by B. subtilis (Figure 5). Log reduction of 0.257 and % reduction of 44.7% was seen in B. subtilis after MB treatment, which is much less than that of the reduction in E. coli and D. radiodurans bacterial count under similar conditions.
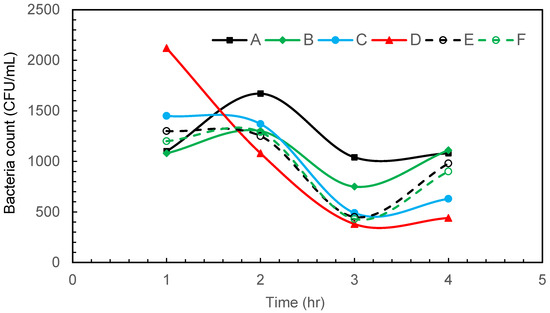
Figure 5.
Intermittent microbubbling of B. subtilis contaminated water with 6 sampling points in tank.
The comparatively lower reduction in B. subtilis is consistent with its known structural defences. As a gram-positive bacterium, it possesses a thick, multilayered peptidoglycan cell wall, which offers mechanical protection against shear stress and reactive oxygen species generated during microbubble collapse [25,28]. These structural attributes are likely to reduce the efficiency of disinfection relative to E. coli and D. radiodurans, both of which lack such robust wall architecture. While no ultrastructural analysis was performed here, the observed disinfection trend aligns with cell envelope theory and supports the role of wall composition in modulating bacterial susceptibility.
Although air microbubble treatment did not fully eliminate the bacteria in the water, a reduction of approximately 45% in potentially harmful bacteria may be effective in preventing disease outbreaks. The difference in the cell wall structure of microorganisms can be the cause of the differences in the elimination rate of different species of bacteria. Physical and chemical breakage of the cell walls is thought to be the main mechanism causing bacterial death under hydrodynamic cavitation [29]. Gram-positive bacteria have thick peptidoglycan layers covering their cell walls, along with trace levels of teichoic acids. Gram-negative bacteria have a thinner peptidoglycan layer than gram-positive bacteria [30] and hence it is anticipated that they will be less resistant to cavitation-induced cell wall rupture. In general, disinfection rates for gram-negative species were greater compared to gram-positive species.
The results presented in this study are based on synthetically prepared contaminated water samples under controlled laboratory conditions. The practical applicability of a microbubble-based disinfection system requires further investigation to sustain the effectiveness of the approach in real wastewater treatment scenarios. In natural or real wastewater, the presence of organic matter, suspended solids, and dissolved ions (chloride, sulphates, or calcium) compete with the microbial cell walls for the reactive oxygen species and may become preferentially oxidised, potentially decreasing the disinfection efficiency of the system. Additionally, the presence of turbidity or suspended solids in real wastewater may hinder the physical or chemical interaction of microbubbles with harmful microorganisms. However, the cooperative physical (shear stress and cavitation) and chemical (generation of reactive species) effects associated with microbubbles will help mitigate the influence of different contaminants. A comparative study of real and synthetic water with a varying concentration of organic and inorganic contaminants is recommended for future study to evaluate the practical applicability of the system.
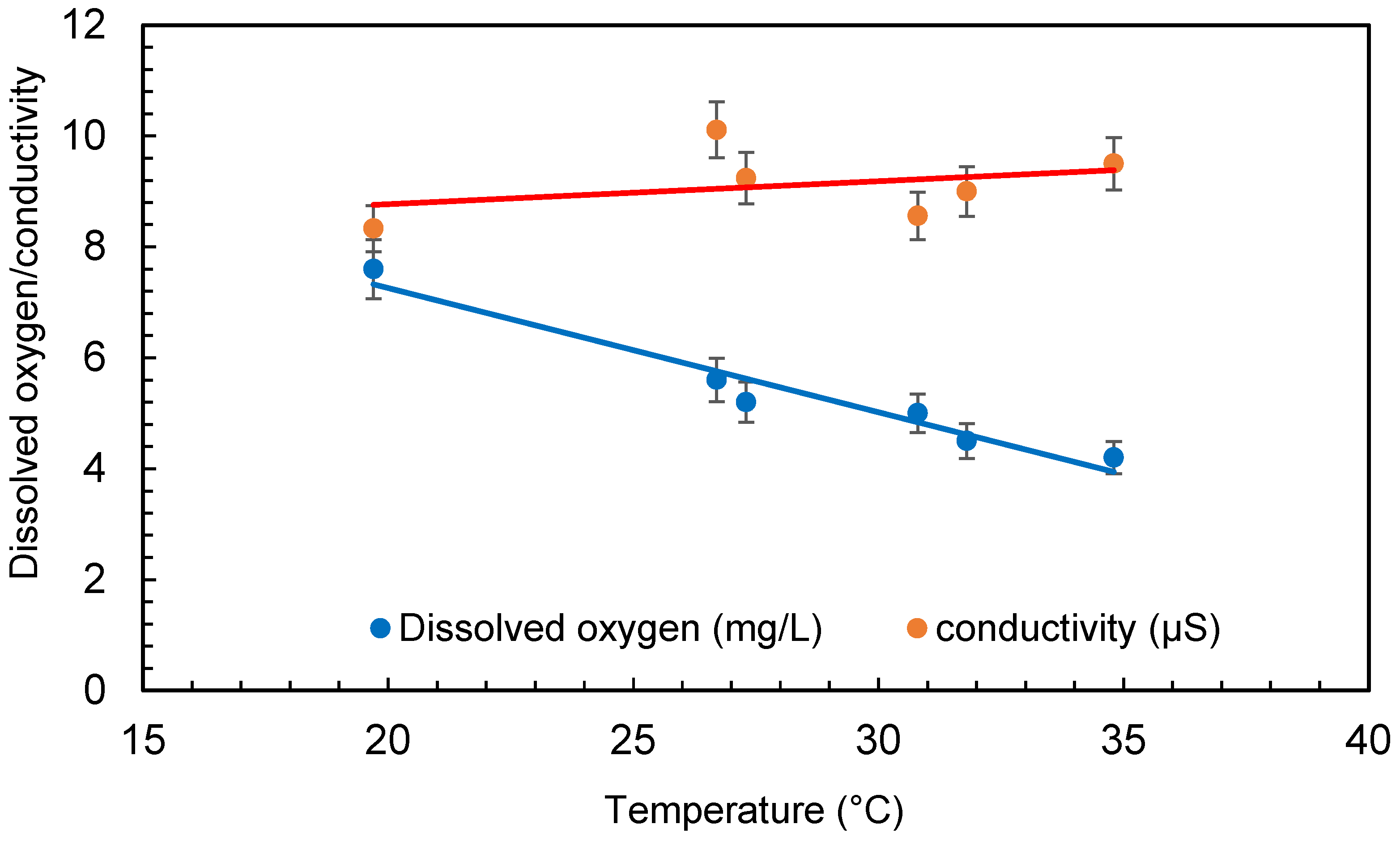
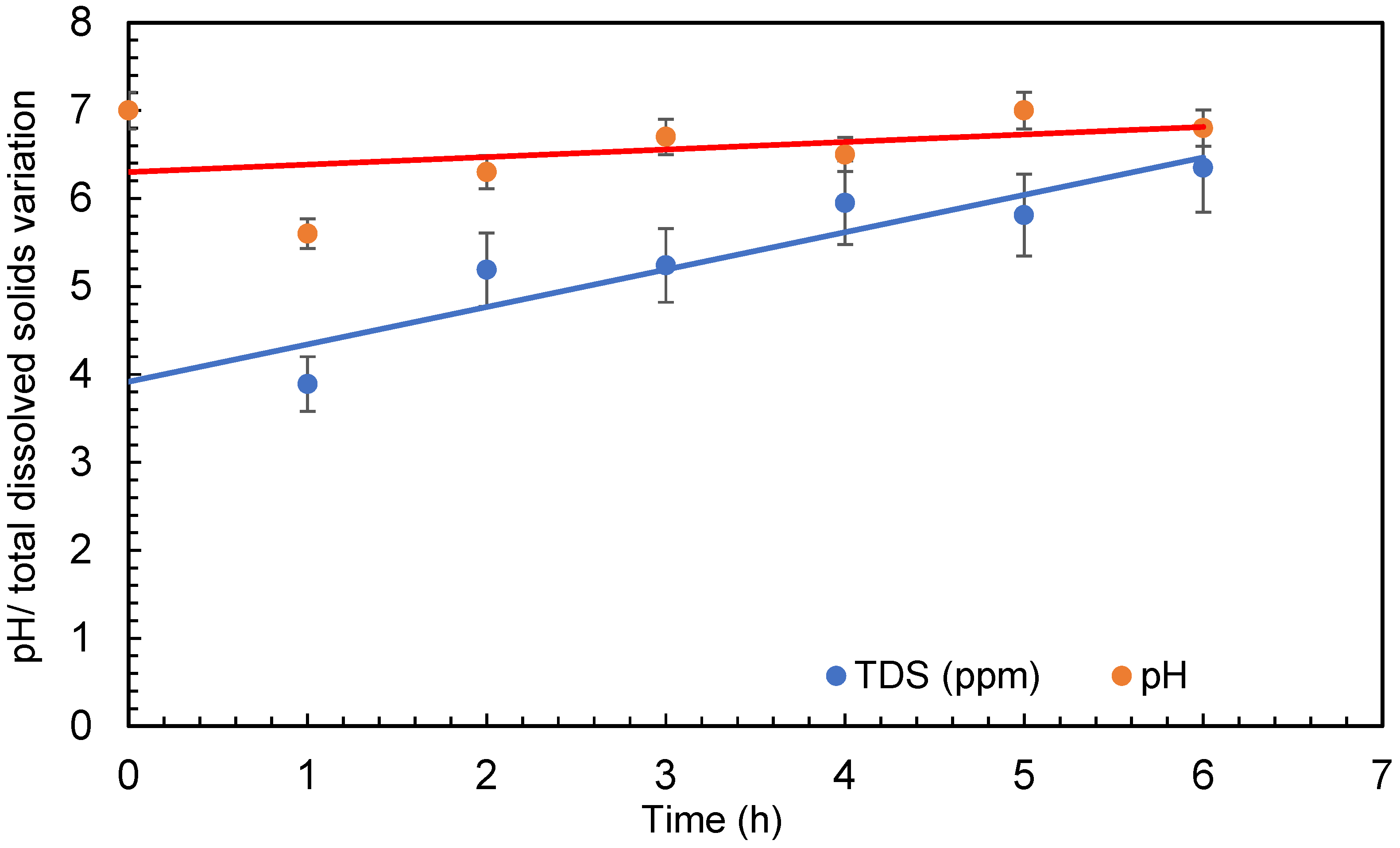
The temperature of the water was raised significantly throughout several treatments in the small amount of water (30 L) taken for the experimental procedure. The temperature of the water was raised from 20 ± 1 °C to 35 ± 1 °C during the 6 h of treatment. The pump of the microbubble generator generates a significant quantity of heat during operation, which is transferred to the water. However, due to the quick exchange of temperature between the water body and the surrounding air, this may not be a problem with large quantities of water. The increase in temperature has no effect on bacteria growth as it is within the range of the temperature for each bacteria growth. The range of temperature for Escherichia coli (E. coli) is 7–50 °C, Bacillus subtilis is 18–43 °C, and Deinococcus radiodurans is 20–42 °C. Dissolved oxygen first increased at the beginning of the experiment and then started decreasing as the treatment continued. Dissolved oxygen was 5.8 ± 0.5 mg/L in the beginning and increased to 7.6 ± 0.5 mg/L after 4 min of microbubbling. Then, the dissolved oxygen continued to decrease through the experiment. The conductivity of the water was found to increase during the treatment (seen in Figure 6), which can be due to the dispersion of the solids caused by microbubbling and to the increase in temperature of the bubbled water. Figure 7 presents the conductivity and the total dissolved solids. It can be observed that an increase in conductivity correlates well with the increase in total dissolved solids (TDS) presented in Figure 6. A similar trend was observed for TDS as reported by Hellar-Kihampa et al. [31]. On the other hand, the pH of the water did not change much before and after the microbubble treatment and was within the error limit of the experiment.
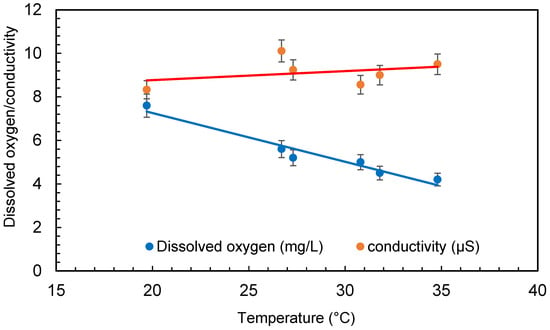
Figure 6.
Conductivity and dissolved oxygen variation during treatment (error bars represents error percentages from 3 replicate experiments).
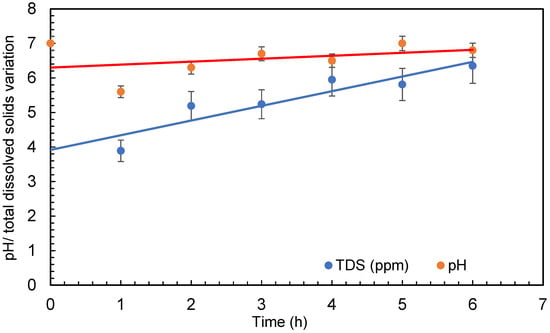
Figure 7.
pH and total dissolved solids variation during treatment (error bars represents error percentages from 3 replicate experiments).
Our initial findings of disinfection efficacy in the model contaminated water with air microbubbling suggest a promising non-reagent low-cost greener disinfection technology to control the growth of gram-negative, gram-positive, and radiant-resistant bacteria. One of the limitations of this study is that the trials were conducted in deionised water and tested bacteria were introduced into the deionised water in the tank just before the trial. Another possibility is that the studies could be conducted at different bacterial loads (high and low bacterial concentrations) to establish the exact mechanism of the microbubble disinfection. So, a comprehensive investigation is required prior to the scaling up of the air microbubble technology for commercial applications regarding the evaluation of the contaminated water sample with different bacterial loads.
4. Conclusions
Microbubble technology is effective for treating water contaminated with different types of bacteria. The microbial population was significantly lowered in air microbubble-treated water. MB treatment can reduce E. coli, D. radiodurans, and B. subtilis with 1.36, 5, and 0.257 log reduction, respectively, relating to a percentage reduction of 95.7%, 100%, and 45%, respectively, after the treatment. In general, disinfection rates for gram-negative and radiation-resistant species were greater compared to gram-positive species. The differences in the elimination rate of different species of bacteria by the air microbubbles are attributed to the difference in the cell wall structure of different microorganisms. A decrease in dissolved oxygen (DO) and an increase in conductivity is observed during the treatment correlating to an increase in the total dissolved solids (TDS) seen during the experiment with microbubble dispersion. Although air microbubble treatment did not eliminate all types of bacteria in the water, a reduction of 45% in gram-positive and 100% disinfection in radiation-resistant harmful bacteria may be effective in preventing disease outbreaks. This non-chemical disinfection method might offer a promising antibiotic substitute.
Regarding the sampling technique, the results in Figure 3, Figure 4 and Figure 5 show no increase in bacterial concentration at the bottom of the tank (sampling point d). Also, the trends observed at all sampling locations in each figure are almost similar. These results indicate that the sampling approach was effective in demonstrating homogeneity in bacterial distribution in the tank.
In future studies, we will consider the following improvements: (i) a flow-through system to simulate more realistic hydraulic conditions, evaluate the impact on disinfection performance, and optimise treatment duration for practical deployment will be considered; (ii) spargers or diffusers will be incorporated to enhance the homogeneous distribution of bubbles and increase the likelihood of microbubble–bacteria contact; and (iii) we will also consider using computational fluid dynamics (CFD) simulations to better understand flow patterns and optimise microbubble distribution.
Author Contributions
Conceptualization, F.H., R.P.-C., S.J.D., T.J.S. and P.B.G.; methodology, F.H., S.S.N. and S.J.D.; formal analysis, S.S.N.; investigation, S.S.N.; resources, S.J.D., T.J.S. and P.B.G.; data curation, S.S.N.; writing—original draft preparation, S.S.N.; writing—review and editing, F.H., S.S.N. and S.J.D.; visualization, S.S.N.; supervision, F.H., R.P.-C. and S.J.D.; project administration, F.H., R.P.-C. and S.J.D.; funding acquisition, F.H., R.P.-C., S.J.D. and T.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the ‘THYME project’ programme.. This project also received expert guidance from Veolia Water Technologies.
Institutional Review Board Statement
An ethics approval statement is not applicable.
Data Availability Statement
Data available on request.
Conflicts of Interest
Anthony John Stubbs is from Water Core Ltd. The other authors declare no conflict of interest.
References
- Zupanc, M.; Pandur, Ž.; Perdih, T.S.; Stopar, D.; Petkovšek, M.; Dular, M. Effects of cavitation on different microorganisms: The current understanding of the mechanisms taking place behind the phenomenon. A review and proposals for further research. Ultrason. Sonochem. 2019, 57, 147–165. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 3rd ed.; WHO Press: Geneva, Switzerland, 2004. [Google Scholar]
- Cotruvo, J.A.; Amato, H. Trihalomethanes: Concentrations, cancer risks, and regulations. J.-Am. Water Work. Assoc. 2019, 111, 12–20. [Google Scholar] [CrossRef]
- Gogate, P.R. Application of cavitational reactors for water disinfection: Status 22 and path forward. J. Environ. Manag. 2007, 85, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Pinedo-Cuenca, R.; Stubbs, T.; Davis, S.J.; Ganesan, P.B.; Hamad, F. Contemporary application of microbubble technology in water treatment. Water Sci. Technol. 2022, 86, 2138–2156. [Google Scholar] [CrossRef]
- Kirti, S.; Bhandari, V.M.; Jena, J.; Sorokhaibam, L.G.; Bhattacharyya, A.S. Exploiting functionalities of biomass in nanocomposite development: Application in dye removal and disinfection along with process intensification. Clean Technol. Environ. Policy 2018, 20, 981–994. [Google Scholar] [CrossRef]
- Cerecedo, L.M.; Dopazo, C.; Gomez-Lus, R. Water disinfection by hydrodynamic cavitation in a rotor-stator device. Ultrason. Sonochem. 2018, 48, 71–78. [Google Scholar] [CrossRef]
- Mancuso, G.; Langone, M.; Andreottola, G. A critical review of the current technologies in wastewater treatment plants by using hydrodynamic cavitation process: Principles and applications. J. Environ. Health Sci. Eng. 2020, 18, 311–333. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Z.; Xuan, X.; Ji, L.; Li, X.; Tao, Y.; Boczkaj, G.; Zhao, S.; Yoon, J.Y.; Chen, S. Disinfection characteristics of an advanced rotational hydrodynamic cavitation reactor in pilot scale. Ultrason. Sonochem. 2021, 73, 105543. [Google Scholar] [CrossRef]
- Nair, S.; Marasini, R.; Buck, L.; Dhodapkar, R.; Marugán, J.; Lakshmi, K.; Mcguigan, K. Life cycle assessment comparison of point-of-use water treatment technologies: Solar water disinfection (SODIS), boiling water, and chlorination. J. Environ. Chem. Eng. 2023, 11, 110015. [Google Scholar] [CrossRef]
- Jhunkeaw, C.; Khongcharoen, N.; Rungrueng, N.; Sangpo, P.; Panphut, W.; Thapinta, A.; Senapin, S.; St-Hilaire, S.; Dong, H.T. Ozone nanobubble treatment in freshwater effectively reduced pathogenic fish bacteria and is safe for Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 534, 736286. [Google Scholar] [CrossRef]
- Tamaki, M.; Kobayashi, F.; Ikeura, H.; Sato, M. Disinfection by ozone microbubbles can cause morphological change of Fusarium oxysporum f. sp. melonis spores. Plant Pathol. J. 2018, 34, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xi, J.; Huang, J.-J.; Hu, H.-Y. Effect of inlet ozone concentration on the performance of a micro-bubble ozonation system for inactivation of Bacillus subtilis spores. Sep. Purif. Technol. 2013, 114, 126–133. [Google Scholar] [CrossRef]
- Mezule, L.; Tsyfansky, S.; Yakushevich, V.; Juhna, T. A simple technique for water disinfection with hydrodynamic cavitation: Effect on survival of Escherichia coli. Desalination 2010, 251, 152–159. [Google Scholar] [CrossRef]
- Kobayashi, F.; Hayata, Y.; Ikeura, Y.; Tamaki, M.; Muto, N.; Osajima, Y. Inactivation of Escherichia coli by CO microbubbles at a lower pressure and near room temperature. Am. Soc. Agric. Eng. 2009, 52, 1621–1626. [Google Scholar]
- Jain, P.; Bhandari, V.M.; Balapure, K.; Jena, J.; Ranade, V.V.; Killedar, D.J. Hydrodynamic cavitation using vortex diode: An efficient approach for elimination of pathogenic bacteria from water. J. Environ. Manag. 2019, 242, 210–219. [Google Scholar] [CrossRef]
- Basso, A.; Hamad, F.; Ganesan, P.B. Initial Results from the Experimental and Computational Study of Microbubble Generation. In Proceedings of the 4th World Congress on Momentum, Heat and Mass Transfer (MHMT’19), Rome, Italy, 10–12 April 2019. [Google Scholar]
- Hamad, F.A.; Pun, K.; Alessio, B.; Najim, S.A.; Ganesan, P.B.; Hughes, D. Experimental measurements on the microbubble characteristics and dissolved oxygen (DO) in water using single and twin-Venturi type microbubble generators. Chem. Eng. Sci. 2023, 280, 118994. [Google Scholar] [CrossRef]
- Davis, S.J.; Vener, A.V.; Vierstra, R.D. Bacteriophytochromes: Phytochrome-like photoreceptors from nonphotosynthetic eubacteria. Science 1999, 286, 2517–2520. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef]
- Bai, X.; Nakatsu, C.H.; Bhunia, A.K. Bacterial biofilms and their implications in pathogenesis and food safety. Foods 2021, 10, 2117. [Google Scholar] [CrossRef]
- Setlow, P. Germination of spores of Bacillus species: What we know and do not know. J. Bacteriol. 2014, 196, 1297–1305. [Google Scholar] [CrossRef]
- Haque, M.A.; Wang, F.; Chen, Y.; Hossen, F.; Islam, M.A.; Hossain, M.A.; Siddique, N.; He, C.; Ahmed, F. Bacillus spp. Contamination: A Novel Risk Originated From Animal Feed to Human Food Chains in South-Eastern Bangladesh. Front. Microbiol. 2022, 12, 783103. [Google Scholar] [CrossRef] [PubMed]
- Deeba, F.; Pruthi, V.; Negi, Y.S. Effect of emerging contaminants from paper mill industry into the environment and their control. In Environmental Contaminants; Springer: Berlin/Heidelberg, Germany, 2017; pp. 391–408. [Google Scholar] [CrossRef]
- Kumar, S.; Mollo, A.; Kahne, D.; Ruiz, N. The bacterial cell wall: From Lipid II flipping to polymerization. Chem. Rev. 2022, 122, 8884–8924. [Google Scholar] [CrossRef] [PubMed]
- Bandala, E.R.; Rodriguez-Narvaez, O.M. On the nature of hydrodynamic cavitation process and its application for the removal of water pollutants. Air Soil Water Res. 2019, 12, 1178622119880488. [Google Scholar] [CrossRef]
- Liu, C.; Tang, Y. Application research of micro and nano bubbles in water pollution control. In Proceedings of the 2019 9th International Conference on Biomedical Engineering and Technology, Tokyo, Japan, 28–30 March 2019. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Arrojo, S.; Benito, Y.; Tarifa, A.M. A parametrical study of disinfection with hydrodynamic cavitation. Ultrason. Sonochem. 2008, 15, 903–908. [Google Scholar] [CrossRef]
- Loraine, G.; Chahine, G.; Hsiao, C.T.; Choi, J.K.; Aley, P. Disinfection of gram-negative and gram-positive bacteria using DynaJets® hydrodynamic cavitating jets. Ultrason. Sonochem. 2012, 19, 710–717. [Google Scholar] [CrossRef]
- Hellar-Kihampa, H.; De Wael, K.; Lugwisha, E.; Van Grieken, R. Water quality assessment in the Pangani River basin, Tanzania: Natural and anthropogenic influences on the concentrations of nutrients and inorganic ions. Int. J. River Basin Manag. 2013, 11, 55–75. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).