Persistent Organic Pollutants’ Threats and Impacts on Food Safety in the Polar Regions—A Concise Review
Abstract
1. Introduction
2. Emerging Global Threats of Persistent Organic Pollutants to Foods
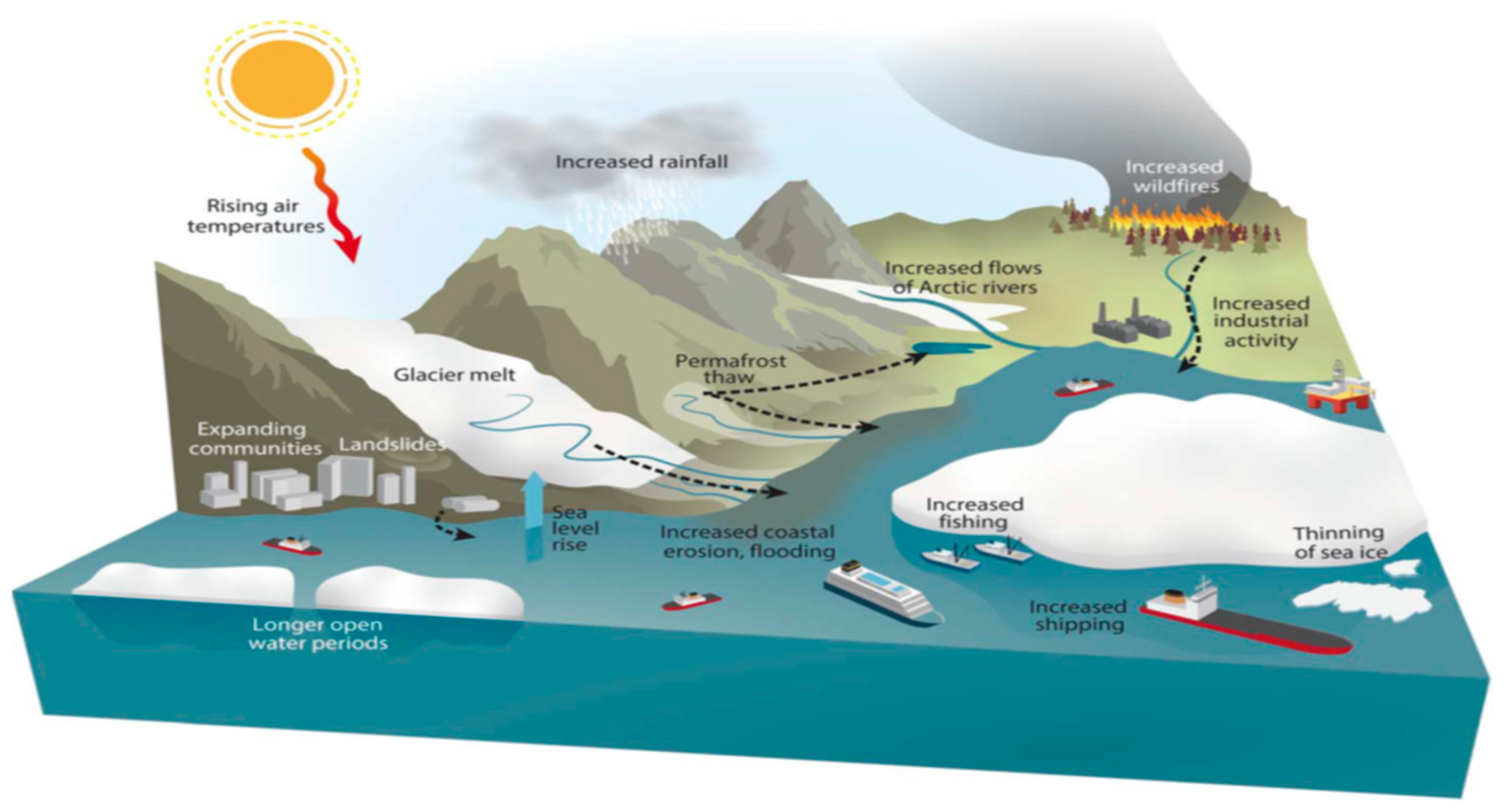
3. Monitoring the Level of Persistent Organic Pollutants in Arctic Traditional Foods
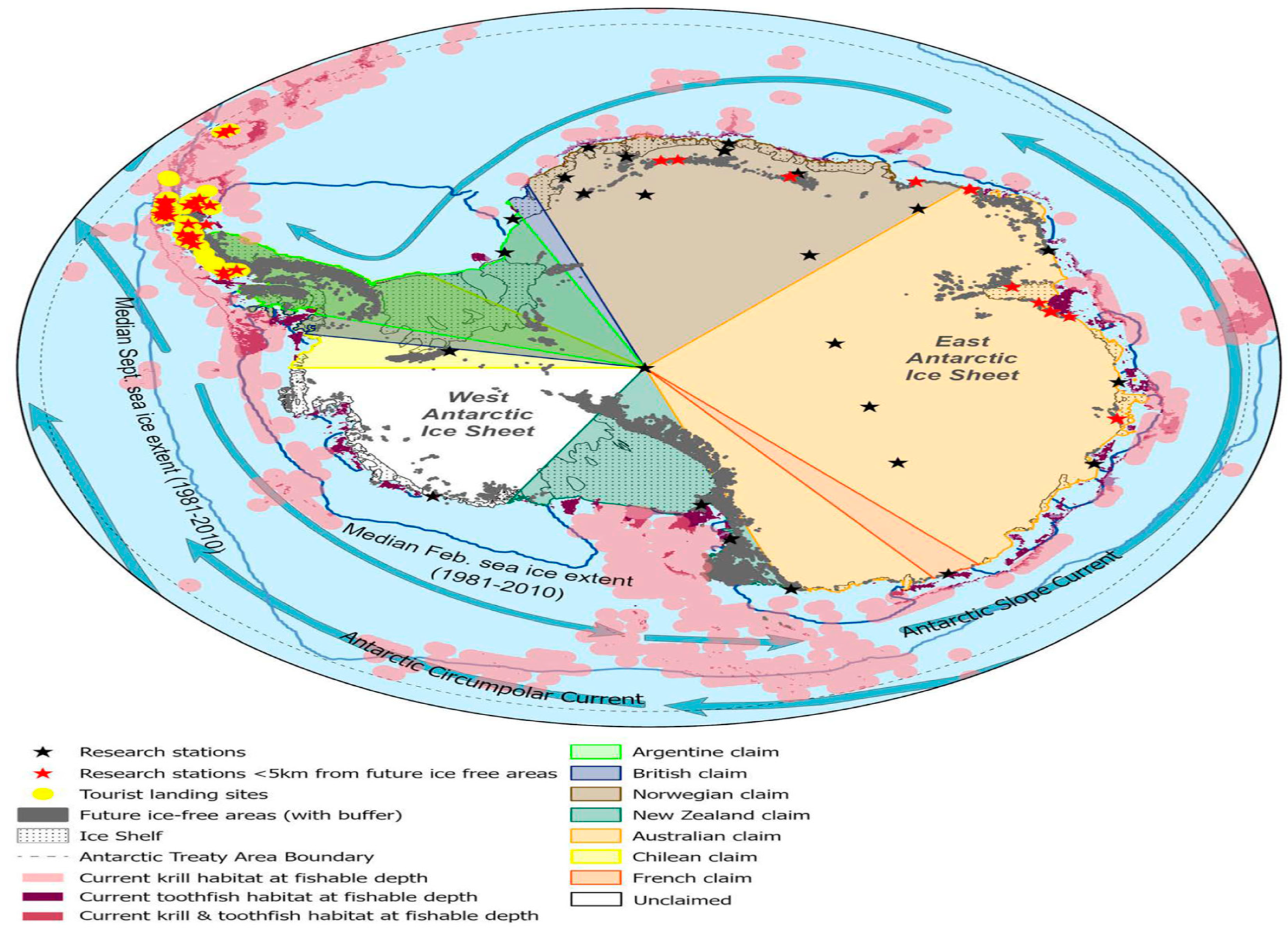
4. Monitoring the Level of Persistent Organic Pollutants in Antarctica
5. Food Security, Sovereignty and Resilience in the Polar Regions
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friedrich, D. The problems won’t go away: Persistent Organic Pollutants (POPs) in the Arctic. The Arctic Institute. Thearcticinstitute.org. 2016. Available online: https://www.thearcticinstitute.org/persistent-organic-pollutants-pops-in-the-arctic/ (accessed on 18 January 2025).
- Ashraf, M.A. Persistent organic pollutants (POPs): A global issue, a global challenge. Environ. Sci. Pollut. Res. 2017, 24, 4223–4227. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.; Herrmann, T.K.; Raheem, D. Food security across the circumpolar region. In Routledge Handbook of Arctic Security; Gunhild, G., Lanteigne, M., Sam-Aggrey, H., Eds.; Taylor Francis Group: Oxfordshire, UK, 2020; pp. 417–426. [Google Scholar]
- UNEP/GPA. The State of the Marine Environment: Trends and Processes; United Nations Environment Programme (UNEP): Hague, The Netherlands, 2006. [Google Scholar]
- UNEP. Persistent Organic Pollutants (POPs) and Pesticides. 2025. Available online: https://www.unep.org/cep/persistent-organic-pollutants-pops-and-pesticides#:~:text=These%20were%20a%20group%20of,%2C%20polychlorinated%20dibenzofurans%2C%20and%20toxaphen (accessed on 10 April 2025).
- EPA—US Environmental Protection Agency. Persistent Organic Pollutants: A Global Issue, A Global Response. 2025. Available online: https://www.epa.gov/international-cooperation/persistent-organic-pollutants-global-issue-global-response (accessed on 6 April 2025).
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H. Persistent Organic Pollutants in Food: Contamination Sources, Health Effects and Detection Methods. Int. J. Environ. Res. Public Health 2019, 16, 4361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carpenter, D. Health effects of persistent organic pollutants: The challenge for the Pacific Basin and for the world. Rev. Environ. Health 2011, 26, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, H.; Li, X.; Zhang, J. Persistent organic pollutants in human milk from primiparae—Correlations, global, regional, and national time-trends. Chemosphere 2022, 313, 137484. [Google Scholar] [CrossRef]
- Huntington, H.P. Disturbance, Feedback and Conservation. In Arctic Biodiversity Assessment; Meltofte, H., Josefson, A.B., Payer, D., Eds.; Status and Trends in Arctic Biodiversity; Arctic Council: Tromsø, Norway, 2013; pp. 628–651. [Google Scholar]
- Wang, D.; Ma, H.; Chen, Z.; Shi, G. Occurrences and possible sources of persistent organic pollutants (POPs) in ice-free area soils in East Antarctica. CATENA 2022, 212, 106083. [Google Scholar] [CrossRef]
- Arctic Council. Arctic Council Secretariat. Arctic Peoples. 2025. Available online: https://arctic-council.org/explore/topics/arctic-peoples/ (accessed on 15 December 2024).
- Mancilla, A. South American claims in Antarctica: Colonial, malgré tout. Polar J. 2022, 12, 22–41. [Google Scholar] [CrossRef]
- Gaur, N.; Narasimhulu, K.; PydiSetty, Y. Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment. J. Clean. Prod. 2018, 198, 1602–1631. [Google Scholar] [CrossRef]
- Kumar, J.A.; Krithiga, T.; Sathish, S.; Renita, A.A.; Prabu, D.; Lokesh, S.; Geetha, R.; Namasivayam, S.K.R.; Sillanpaa, M. Persistent organic pollutants in water resources: Fate, occurrence, characterization and risk analysis. Sci. Total. Environ. 2022, 831, 154808. [Google Scholar] [CrossRef]
- World Health Organization. Food Safety: Persistent Organic Pollutants (POPs). Who.int. 2020. Available online: https://www.who.int/news-room/questions-and-answers/item/food-safety-persistent-organic-pollutants-(pops) (accessed on 19 March 2025).
- Stockholm Convention on Persistent Organic Pollutants (POPs). What are POPs? Chm.pops.int. 2024. Available online: https://chm.pops.int/TheConvention/ThePOPs/tabid/673/Default.aspx (accessed on 12 March 2025).
- Bernard, A.; Hermans, C.; Broeckaert, F.; De Poorter, G.; De Cock, A.; Houins, G. Food contamination by PCBs and dioxins. Nature 1999, 401, 231–232. [Google Scholar] [CrossRef]
- Chung, S.W.; Chen, B.L. Determination of organochlorine pesticide residues in fatty foods: A critical review on the analytical methods and their testing capabilities. J. Chromatogr. A 2011, 1218, 5555–5567. [Google Scholar] [CrossRef]
- Costopoulou, D.; Vassiliadou, I.; Leondiadis, L. Infant dietary exposure to dioxins and dioxin-like compounds in Greece. Food Chem. Toxicol. 2013, 59, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L. Concentrations of environmental organic contaminants in meat and meat products and human dietary exposure: A review. Food Chem. Toxicol. 2017, 107, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Gerig, B.S.; Chaloner, D.T.; Janetski, D.J.; Rediske, R.R.; O’keefe, J.P.; Moerke, A.H.; Lamberti, G.A. Congener patterns of persistent organic pollutants establish the extent of contaminant biotransport by pacific salmon in the great lakes. Environ. Sci. Technol. 2015, 50, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Portolés, T.; Sales, C.; Abalos, M.; Sauló, J.; Abad, E. Evaluation of the capabilities of atmospheric pressure chemical ionization source coupled to tandem mass spectrometry for the determination of dioxin-like polychlorobiphenyls in complex-matrix food samples. Anal. Chim. Acta 2016, 937, 96–105. [Google Scholar] [CrossRef]
- Zhao, R.; Chu, S.; Zhao, R.; Xu, X.; Liu, X. Ultrasonic extraction followed by sulfuric acid silica gel cleanup for the determination of α-hexachlorocyclohexane enantiomers in biota samples. Anal. Bioanal. Chem. 2005, 381, 1248–1252. [Google Scholar] [CrossRef]
- Archer, J.C.; Jenkins, R.G. Automated milk fat extraction for the analyses of persistent organic pollutants. J. Chromatogr. B 2017, 1041–1042, 70–76. [Google Scholar] [CrossRef]
- Jensen, E.; Bolger, P.M. Exposure assessment of dioxins/furans consumed in dairy foods and fish. Food Addit. Contam. 2001, 18, 395–403. [Google Scholar] [CrossRef]
- Polder, A.; Savinova, T.; Tkachev, A.; Løken, K.; Odland, J.; Skaare, J. Levels and patterns of Persistent Organic Pollutants (POPS) in selected food items from Northwest Russia (1998–2002) and implications for dietary exposure. Sci. Total. Environ. 2010, 408, 5352–5361. [Google Scholar] [CrossRef]
- Thompson, L.A.; Darwish, W.S. Environmental Chemical Contaminants in Food: Review of a Global Problem. J. Toxicol. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Patel, K.; Fussell, R.J.; Hetmanski, M.; Goodall, D.M.; Keely, B.J. Evaluation of gas chromatography–tandem quadrupole mass spectrometry for the determination of organochlorine pesticides in fats and oils. J. Chromatogr. A 2005, 1068, 289–296. [Google Scholar] [CrossRef]
- Stefanelli, P.; Santilio, A.; Cataldi, L.; Dommarco, R. Multiresidue analysis of organochlorine and pyrethroid pesticides in ground beef meat by gas chromatography-mass spectrometry. J. Environ. Sci. Health Part B 2009, 44, 350–356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Shahawi, M.; Hamza, A.; Bashammakh, A.; Al-Saggaf, W. An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta 2010, 80, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lu, Y.; Han, J.; Luo, W.; Shi, Y.; Wang, T.; Sun, Y. Hexachlorobenzene sources, levels and human exposure in the environment of China. Environ. Int. 2010, 36, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-S.; Sthiannopkao, S.; Du, J.; Chen, Z.-J.; Kim, K.-W.; Yasin, M.S.M.; Hashim, J.H.; Wong, C.K.-C.; Wong, M.-H. Daily intake and human risk assessment of organochlorine pesticides (OCPs) based on Cambodian market basket data. J. Hazard. Mater. 2011, 192, 1441–1449. [Google Scholar] [CrossRef]
- Tesi, G.O.; Okpara, K.E.; Tesi, J.N.; Agbozu, I.E.; Techato, K. Human exposure to endocrine-disrupting organochlorine and organophosphate pesticides in locally produced and imported honey in Nigeria. Int. J. Environ. Health Res. 2024, 35, 784–804. [Google Scholar] [CrossRef]
- Kedikoglou, K.; Costopoulou, D.; Vassiliadou, I.; Bakeas, E.; Leondiadis, L. An effective and low-cost carbon-based clean-up method for PCDD/Fs and PCBs analysis in food. Chemosphere 2018, 206, 531–538. [Google Scholar] [CrossRef]
- Čajka, T.; Hajšlová, J.; Kazda, R.; Poustka, J. Challenges of gas chromatography–high-resolution time-of-flight mass spectrometry for simultaneous analysis of polybrominated diphenyl ethers and other halogenated persistent organic pollutants in environmental samples. J. Sep. Sci. 2005, 28, 601–611. [Google Scholar] [CrossRef]
- Chan, H.M.; El Khoury, M.; Sedgemore, M.; Sedgemore, S.; Kuhnlein, H.V. Organochlorine pesticides and polychlorinated biphenyl congeners in Ooligan grease: A traditional food fat of British Columbia First Nations. J. Food Compos. Anal. 1996, 9, 32–42. [Google Scholar] [CrossRef]
- Megson, D.; Reiner, E.J.; Jobst, K.J.; Dorman, F.L.; Robson, M.; Focant, J.-F. A review of the determination of persistent organic pollutants for environmental forensics investigations. Anal. Chim. Acta 2016, 941, 10–25. [Google Scholar] [CrossRef]
- Shoiful, A.; Fujita, H.; Watanabe, I.; Honda, K. Concentrations of organochlorine pesticides (OCPs) residues in foodstuffs collected from traditional markets in Indonesia. Chemosphere 2013, 90, 1742–1750. [Google Scholar] [CrossRef]
- Tang, H.P.-O. Recent development in analysis of persistent organic pollutants under the Stockholm Convention. TrAC Trends Anal. Chem. 2013, 45, 48–66. [Google Scholar] [CrossRef]
- Usydus, Z.; Szlinder-Richert, J.; Polak-Juszczak, L.; Komar, K.; Adamczyk, M.; Malesa-Ciecwierz, M.; Ruczynska, W. Fish products available in Polish market–Assessment of the nutritive value and human exposure to dioxins and other contaminants. Chemosphere 2009, 74, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Herold, C.; Hollert, H.; Kamphues, J.; Blepp, M.; Ballschmiter, K. Reviewing the relevance of dioxin and PCB sources for food from animal origin and the need for their inventory, control and management. Environ. Sci. Eur. 2018, 30, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, M.D.; Martin, M.; Tjeerdema, R.S. Long-term trends in DDT, polychlorinated biphenyls, and chlordane in California mussels. Arch. Environ. Contam. Toxicol. 1995, 28, 443–450. [Google Scholar] [CrossRef]
- Tadeo, J.L.; Sánchez-Brunete, C.; Albero, B.; García-Valcárcel, A.I. Application of ultrasound-assisted extraction to the determination of contaminants in food and soil samples. J. Chromatogr. A 2010, 1217, 2415–2440. [Google Scholar] [CrossRef]
- Corsolini, S.; Ademollob, N. POPs in Antarctic ecosystems: Is climate change affecting their temporal trends? Environ. Sci. Process. Impacts 2022, 24, 1631–1642. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, P.; Wu, Z.; Zhang, S.; Wei, L.; Mi, L.; Kuester, A.; Gandrass, J.; Ebinghaus, R.; Yang, R.; et al. Legacy and emerging organic contaminants in the polar regions. Sci. Total. Environ. 2022, 835, 155376. [Google Scholar] [CrossRef]
- Luarte, T.; Gómez-Aburto, V.A.; Poblete-Castro, I.; Castro-Nallar, E.; Huneeus, N.; Molina-Montenegro, M.; Egas, C.; Azcune, G.; Pérez-Parada, A.; Lohmann, R.; et al. Levels of persistent organic pollutants (POPs) in the Antarctic atmosphere over time (1980 to 2021) and estimation of their atmospheric half-lives. Atmos. Meas. Tech. 2023, 23, 8103–8118. [Google Scholar] [CrossRef]
- Mangano, M.C.; Sarà, G.; Corsolini, S. Monitoring of persistent organic pollutants in the polar regions: Knowledge gaps gluts through evidence mapping. Chemosphere 2016, 172, 37–45. [Google Scholar] [CrossRef]
- Esteban, S.; Moreno-Merino, L.; Matellanes, R.; Catalá, M.; Gorga, M.; Petrovic, M.; de Alda, M.L.; Barceló, D.; Silva, A.; Durán, J.; et al. Presence of endocrine disruptors in freshwater in the northern Antarctic Peninsula region. Environ. Res. 2016, 147, 179–192. [Google Scholar] [CrossRef]
- Mwangi, J.K.; Lee, W.-J.; Wang, L.-C.; Sung, P.-J.; Fang, L.-S.; Lee, Y.-Y.; Chang-Chien, G.-P. Persistent organic pollutants in the Antarctic coastal environment and their bioaccumulation in penguins. Environ. Pollut. 2016, 216, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Roscales, J.L.; Muñoz-Arnanz, J.; Barbosa, A.; Jiménez, B. Evaluation of PCDD/Fs, PCBs and PBDEs in two penguin species from Antarctica. Chemosphere 2021, 286, 131871. [Google Scholar] [CrossRef] [PubMed]
- Vecchiato, M.; Argiriadis, E.; Zambon, S.; Barbante, C.; Toscano, G.; Gambaro, A.; Piazza, R. Persistent Organic Pollutants (POPs) in Antarctica: Occurrence in continental and coastal surface snow. Microchem. J. 2014, 119, 75–82. [Google Scholar] [CrossRef]
- Nash, S.B.; Bohlin-Nizzetto, P.; Galban-Malagon, C.; Corsolini, S.; Cincinelli, A.; Lohmann, R. Monitoring persistent organic chemicals in Antarctica in support of global chemical policy: A horizon scan of priority actions and challenges. Lancet Planet. Health 2023, 7, e435–e440. [Google Scholar] [CrossRef]
- Alharbi, O.M.; Basheer, A.A.; Khattab, R.A.; Ali, I. Health and environmental effects of persistent organic pollutants. J. Mol. Liq. 2018, 263, 442–453. [Google Scholar] [CrossRef]
- Wang, S.-L.; Tsai, P.-C.; Yang, C.-Y.; Guo, Y.L. Increased risk of diabetes and polychlorinated biphenyls and dioxins. Diabetes Care 2008, 31, 1574–1579. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Consultation on assessment of the health risk of dioxins; re-evaluation of the tolerable daily intake (TDI): Executive Summary. Food Addit. Contam. 2000, 17, 223–240. [Google Scholar] [CrossRef]
- WHO. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA)—Polybrominated Diphenyl Ethers (PBDEs). 2006. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/5294#:~:text=The%20large%20margin%20of%20exposure,be%20a%20significant%20health%20concern (accessed on 2 April 2025).
- Li, Q.Q.; Loganath, A.; Chong, Y.S.; Tan, J.; Obbard, J.P. Persistent organic pollutants and adverse health effects in humans. J. Toxicol. Environ. Health Part A 2006, 69, 1987–2005. [Google Scholar] [CrossRef]
- Zong, G.; Valvi, D.; Coull, B.; Göen, T.; Hu, F.B.; Nielsen, F.; Grandjean, P.; Sun, Q. Persistent organic pollutants and risk of type 2 diabetes: A prospective investigation among middle-aged women in Nurses’ Health Study II. Environ. Int. 2018, 114, 334–342. [Google Scholar] [CrossRef]
- Multigner, L.; Kadhel, P.; Rouget, F.; Blanchet, P.; Cordier, S. Chlordecone exposure and adverse effects in French West Indies populations. Environ. Sci. Pollut. Res. 2015, 23, 3–8. [Google Scholar] [CrossRef]
- Hernández, Á.R.; Boada, L.D.; Mendoza, Z.; Ruiz-Suárez, N.; Valerón, P.F.; Camacho, M.; Zumbado, M.; Almeida-González, M.; Henríquez-Hernández, L.A.; Luzardo, O.P. Consumption of organic meat does not diminish the carcinogenic potential associated with the intake of persistent organic pollutants (POPs). Environ. Sci. Pollut. Res. 2015, 24, 4261–4273. [Google Scholar] [CrossRef] [PubMed]
- Sonne, C.; Gustavson, K.; Bossi, R.; Søndergaard, J.; Desforges, J.-P.; Bonefeld-Jørgensen, E.C.; Dietz, R. Ubiquitous global use of persistent PFAS threatens Arctic Indigenous peoples for decades to come. Cell Rep. Sustain. 2025, 2, 100341. [Google Scholar] [CrossRef]
- Burgoon, L.D.; Clewell, H.J.; Cox, T.; Dekant, W.; Dell, L.D.; Deyo, J.A.; Dourson, M.L.; Gadagbui, B.K.; Goodrum, P.; Green, L.C.; et al. Range of the perfluorooctanoate (PFOA) safe dose for human health: An international collaboration. Regul. Toxicol. Pharmacol. 2023, 145, 105502. [Google Scholar] [CrossRef] [PubMed]
- Lyche, J.L.; Rosseland, C.; Berge, G.; Polder, A. Human health risk associated with brominated flame-retardants (BFRs). Environ. Int. 2014, 74, 170–180. [Google Scholar] [CrossRef]
- Roosens, L.; Abdallah, M.A.-E.; Harrad, S.; Neels, H.; Covaci, A. Exposure to Hexabromocyclododecanes (HBCDs) via Dust Ingestion, but Not Diet, Correlates with Concentrations in Human Serum: Preliminary Results. Environ. Health Perspect. 2009, 117, 1707–1712. [Google Scholar] [CrossRef]
- EFSA Panel name on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; et al. Risks for animal and human health related to the presence of polychlorinated naphthalenes (PCNs) in feed and food. EFSA J. 2024, 22, e8640. [Google Scholar]
- Kanan, S.; Samara, F. Dioxins and furans: A review from chemical and environmental perspectives. Trends Environ. Anal. Chem. 2017, 17, 1–13. [Google Scholar] [CrossRef]
- Hao, Y.; Li, Y.; Han, X.; Wang, T.; Yang, R.; Wang, P.; Xiao, K.; Li, W.; Lu, H.; Fu, J.; et al. Air monitoring of polychlorinated biphenyls, polybrominated diphenyl ethers and organochlorine pesticides in West Antarctica during 2011–2017: Concentrations, temporal trends and potential sources. Environ. Pollut. 2019, 249, 381–389. [Google Scholar] [CrossRef]
- Unc, A.; Najm, M.R.A.; Aspholm, P.E.; Bolisetti, T.; Charles, C.; Datta, R.; Eggen, T.; Flem, B.; Hailu, G.; Heimstad, E.S.; et al. Arctic food and energy security at the crossroads. Commun. Earth Environ. 2025, 6, 121. [Google Scholar] [CrossRef]
- Kenny, T. Climate change, contaminants, and country food: Collaborating with communities to promote food security in the Arctic. In Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 2019; pp. 249–263. [Google Scholar] [CrossRef]
- Arctic Council. About the Arctic Council. Arctic-council.org. 2024. Available online: https://arctic-council.org/about/ (accessed on 15 February 2025).
- Arctic Council. Arctic Monitoring and Assessment Programme. Arctic-council.org. 2024. Available online: https://arctic-council.org/about/working-groups/amap/ (accessed on 15 February 2025).
- Reiersen, L.-O.; Vorkamp, K.; Kallenborn, R. The role of the Arctic Monitoring and Assessment Programme (AMAP) in reducing pollution of the Arctic and around the globe. Environ. Sci. Ecotechnol. 2024, 17, 100302. [Google Scholar] [CrossRef]
- UNECE. Protocol to the 1979 Convention on Long-Range Transboundary Air Pollution on Persistent Organic Pollutants. Unece.org. 1998. Available online: http://www.unece.org/env/lrtap/pops_h1.html (accessed on 16 January 2025).
- EUR-Lex. Geneva Convention on Long-Range Transboundary Air Pollution. Eur-lex.europa.eu. 2020. Available online: https://eur-lex.europa.eu/EN/legal-content/summary/geneva-convention-on-long-range-transboundary-air-pollution.html (accessed on 17 January 2025).
- Stockholm Convention on Persistent Organic Pollutants (POPs). Overview. Chm.pops.int. 2024. Available online: https://chm.pops.int/TheConvention/Overview/tabid/3351/Default.aspx (accessed on 26 January 2025).
- Stockholm Convention on Persistent Organic Pollutants (POPs). The 12 Initial POPs under the Stockholm Convention. Chm.pops.int. 2024. Available online: https://chm.pops.int/TheConvention/ThePOPs/The12InitialPOPs/tabid/296/Default.aspx (accessed on 20 January 2025).
- Stockholm Convention on Persistent Organic Pollutants (POPs). All POPs listed in the Stockholm Convention. Chm.pops.int. 2024. Available online: https://chm.pops.int/TheConvention/ThePOPs/AllPOPs/tabid/2509/Default.aspx (accessed on 22 January 2025).
- EPA. DDT—A Brief History and Status. Epa.gov. 2024. Available online: https://www.epa.gov/ingredients-used-pesticide-products/ddt-brief-history-and-status (accessed on 21 February 2025).
- Kumari, K.; Swamy, S. Dichlorodiphenyltrichloroethane (DDT). In Pollutants of Global Concern: A Comprehensive Overview of Persistent Organic Pollutants; Springer International Publishing: Cham, Switzerland, 2024; pp. 31–48. [Google Scholar]
- UNEP. Minamata Convention on Mercury. Minamataconvention.org. 2021. Available online: https://minamataconvention.org/en/about (accessed on 21 January 2025).
- World Health Organization. WHO Calls for the Phase out of Mercury Fever Thermometers and Blood Pressure Measuring Devices by 2020. Who.int. 2013. Available online: https://www.who.int/news/item/11-10-2013-who-calls-for-the-phase-out-of-mercury-fever-thermometers-and-blood-pressure-measuring-devices-by-2020 (accessed on 22 January 2025).
- Grøntoft, T.; Roux, M.S. Convention on long-range transboundary air pollution. In UN/ECE International Operative Programme on Effects on Materials; Including Historic and Cultural Monuments; Environmental data report; October 2020 to December 2021 NILU Rapport; Norwegian Institute for Air Research (NILU): Kjeller, Norway, 2023. [Google Scholar]
- European Environment Agency. Persistent Organic Pollutant Emissions in Europe. Eea.europa.eu. 2024. Available online: https://www.eea.europa.eu/en/analysis/indicators/persistent-organic-pollutant-emissions-in-europe#:~:text=The%20Air%20Convention’s%201998%20Aarhus,POPs%20at%20the%20international%20level (accessed on 11 March 2025).
- Li, L.; Chen, C.; Li, D.; Breivik, K.; Abbasi, G.; Li, Y.-F. What do we know about the production and release of persistent organic pollutants in the global environment? Environ. Sci. Adv. 2023, 2, 55–68. [Google Scholar] [CrossRef]
- Arthur, C. The end is Nigh for Dangerous DDT. Unido.org. 2024. Available online: https://www.unido.org/stories/end-nigh-dangerous-ddt (accessed on 14 March 2025).
- Stockholm Convention on Persistent Organic Pollutants (POPs). The New POPs Under the Stockholm Convention. Chm.pops.int. 2024. Available online: https://chm.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx (accessed on 17 February 2025).
- Xavier, J.C.; Convey, P. Antarctic: Climate change, fisheries, and governance. In Life Below Water; Springer International Publishing: Cham, Switzerland, 2022; pp. 15–26. [Google Scholar]
- Miller, K.M.; Berg, G.; Churchill, I.K.K.O.; Hamilton, F.; Kandiurin, P.S.; de Meulles, C.; Oman, G.; Lickers, M.; McIvor, N.; Henri, D.A. Coexistence between people and polar bears supports Indigenous knowledge mobilization in wildlife management and research. Commun. Earth Environ. 2025, 6, 74. [Google Scholar] [CrossRef]
- Nash, S.B. Persistent organic pollutants in Antarctica: Current and future research priorities. J. Environ. Monit. 2011, 13, 497–504. [Google Scholar] [CrossRef]
- Lohmann, R.; Muir, D.; Zeng, E.Y.; Bao, L.-J.; Allan, I.J.; Arinaitwe, K.; Booij, K.; Helm, P.; Kaserzon, S.; Mueller, J.F.; et al. Aquatic global passive sampling (AQUA-GAPS) revisited: First steps toward a network of networks for monitoring organic contaminants in the aquatic environment. Environ. Sci. Technol. 2017, 51, 1060–1067. Available online: https://pubs.acs.org/doi/full/10.1021/acs.est.6b05159 (accessed on 12 March 2025). [CrossRef] [PubMed]
- Stoeckl, N.; Adams, V.; Baird, R.; Boothroyd, A.; Costanza, R.; Finau, G.; Fulton, E.A.; Hatton MacDonald, D.; King, M.A.; Kubiszewski, I.; et al. Governance challenges to protect globally important ecosystem services of the Antarctic and Southern Ocean. ICES J. Mar. Sci. 2025, 82, fsae163. [Google Scholar] [CrossRef]
- Guardans, R. (Ed.) Update on the global monitoring plan (GMP) in the UNEP Stockholm Convention on Persistent Organic Pollutants (POPs), technical guidance, data analysis, modeling, assessment and workplan. In Proceedings of the 6th SETAC World Congress/SETAC Europe 22nd Annual Meeting, Berlin, Germany, 20–24 May 2012; SETAC: Brussels, Belgium, 2012. [Google Scholar]
- Nash, S.M.B.; Castrillon, J.; Eisenmann, P.; Fry, B.; Shuker, J.D.; Cropp, R.A.; Dawson, A.; Bignert, A.; Bohlin-Nizzetto, P.; Waugh, C.A.; et al. Signals from the south; humpback whales carry messages of Antarctic seaice ecosystem variability. Glob. Change Biol. 2018, 24, 1500–1510. [Google Scholar] [CrossRef]
- Druskat, A.; Ghosh, R.; Castrillon, J.; Nash, S.M.B. Sex ratios of migrating southern hemisphere humpback whales: A new sentinel parameter of ecosystem health. Mar. Environ. Res. 2019, 151, 104749. [Google Scholar] [CrossRef]
- Bargagli, R.; Rota, E. Environmental contamination and climate change in Antarctic ecosystems: An updated overview. Environ. Sci. Adv. 2024, 3, 543–560. [Google Scholar] [CrossRef]
- Wolschke, H.; Meng, X.-Z.; Xie, Z.; Ebinghaus, R.; Cai, M. Novel flame retardants (N-FRs), polybrominated diphenyl ethers (PBDEs) and dioxin-like polychlorinated biphenyls (DL-PCBs) in fish, penguin, and skua from King George Island, Antarctica. Mar. Pollut. Bull. 2015, 96, 513–518. [Google Scholar] [CrossRef]
- Ebinghaus, R.; Barbaro, E.; Nash, S.B.; de Avila, C.; de Wit, C.A.; Dulio, V.; Felden, J.; Franco, A.; Gandrass, J.; Grotti, M.; et al. Berlin statement on legacy and emerging contaminants in polar regions. Chemosphere 2023, 327, 138530. [Google Scholar] [CrossRef]
- Aronson, R.B.; Thatje, S.; McClintock, J.B.; Hughes, K.A. Anthropogenic impacts on marine ecosystems in Antarctica. Ann. N. Y. Acad. Sci. 2011, 1223, 82–107. [Google Scholar] [CrossRef] [PubMed]
- Tin, T.; Fleming, Z.; Hughes, K.; Ainley, D.; Convey, P.; Moreno, C.; Pfeiffer, S.; Scott, J.; Snape, I. Impacts of local human activities on the Antarctic environment. Antarct. Sci. 2008, 21, 3–33. [Google Scholar] [CrossRef]
- Palmer, T.A.; Klein, A.G.; Sweet, S.T.; Montagna, P.A.; Hyde, L.J.; Wade, T.L.; Pollack, J.B. Anthropogenic effects on the marine environment adjacent to Palmer Station, Antarctica. Antarct. Sci. 2021, 34, 79–96. [Google Scholar] [CrossRef]
- Fuoco, R.; Giannarelli, S.; Wei, Y.; Ceccarini, A.; Abete, C.; Francesconi, S.; Termine, M. Persistent organic pollutants (POPs) at Ross Sea (Antarctica). Microchem. J. 2008, 92, 44–48. [Google Scholar] [CrossRef]
- Bargagli, R. Environmental contamination in Antarctic ecosystems. Sci. Total. Environ. 2008, 400, 212–226. [Google Scholar] [CrossRef]
- Kim, J.-T.; Choi, Y.-J.; Barghi, M.; Kim, J.-H.; Jung, J.-W.; Kim, K.; Kang, J.-H.; Lammel, G.; Chang, Y.-S. Occurrence, distribution, and bioaccumulation of new and legacy persistent organic pollutants in an ecosystem on King George Island, maritime Antarctica. J. Hazard. Mater. 2020, 405, 124141. [Google Scholar] [CrossRef]
- Chen, D.; Hale, R.C.; La Guardia, M.J.; Luellen, D.; Kim, S.; Geisz, H.N. Hexabromocyclododecane flame retardant in Antarctica: Research stations as sources. Environ. Pollut. 2015, 206, 611–618. [Google Scholar] [CrossRef]
- Costa, L.R.; Salvador, M.d.l.L.T.; Pintado-Herrera, M.G.; Albergaria-Barbosa, A.C.; Martins, C.C.; Lourenço, R.A.; Combi, T. Legacy and novel contaminants in surface sediments of Admiralty Bay, Antarctica Peninsula. Sci. Total. Environ. 2024, 951, 175551. [Google Scholar] [CrossRef]
- Domínguez-Morueco, N.; Moreno-Merino, L.; Molins-Delgado, D.; Díaz-Cruz, M.S.; Aznar-Alemany, Ò.; Eljarrat, E.; Farré, M.; López-Martínez, J.; de Alda, M.L.; Silva, A.; et al. Anthropogenic contaminants in freshwater from the northern Antarctic Peninsula region. AMBIO 2020, 50, 544–559. [Google Scholar] [CrossRef]
- Alderton, I.; Palmer, B.R.; Heinemann, J.A.; Pattis, I.; Weaver, L.; Gutiérrez-Ginés, M.J.; Horswell, J.; Tremblay, L.A. The role of emerging organic contaminants in the development of antimicrobial resistance. Emerg. Contam. 2021, 7, 160–171. [Google Scholar] [CrossRef]
- Miao, S.; Zhang, Y.; Yuan, X.; Zuo, J. Antibiotic resistance evolution driven synergistically by antibiotics and typical organic pollutants in antibiotic production wastewater. J. Hazard. Mater. 2024, 483, 136543. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial resistance global report on surveillance: 2014 summary. In Antimicrobial Resistance Global Report on Surveillance: 2014 Summary; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Niveda, S.; Rahiman, O.M.F.; Sreenadh, P.K.; Prasanth, M.L.L. A review on the crisis of antibiotic resistance and the strategies to combat resistance. J. Curr. Res. Sci. Med. 2024, 10, 148–154. [Google Scholar] [CrossRef]
- Tam, H.K.; Wong, C.M.V.L.; Yong, S.T.; Blamey, J.; González, M. Multiple-antibiotic-resistant bacteria from the maritime Antarctic. Polar Biol. 2015, 38, 1129–1141. [Google Scholar] [CrossRef]
- Laganà, P.; Caruso, G.; Corsi, I.; Bergami, E.; Venuti, V.; Majolino, D.; La Ferla, R.; Azzaro, M.; Cappello, S. Do plastics serve as a possible vector for the spread of antibiotic resistance? First insights from bacteria associated to a polystyrene piece from King George Island (Antarctica). Int. J. Hyg. Environ. Health 2018, 222, 89–100. [Google Scholar] [CrossRef]
- Caruso, G.; Azzaro, M.; Dell’acqua, O.; Papale, M.; Giudice, A.L.; Laganà, P. Plastic polymers and antibiotic resistance in an Antarctic environment (Ross Sea): Are we revealing the tip of an iceberg? Microorganisms 2024, 12, 2083. [Google Scholar] [CrossRef]
- Caruso, G.; Rappazzo, A.C.; Maimone, G.; Zappalà, G.; Cosenza, A.; Szubska, M.; Zaborska, A. Svalbard Fjord sediments as a hotspot of functional diversity and a reservoir of antibiotic resistance. Environments 2024, 11, 148. [Google Scholar] [CrossRef]
- Caruso, G.; Papale, M.; Rappazzo, A.C.; Azzaro, M. Culturable Plastisphere from the 75° N Subarctic Transect as a Potential Vector of Pathogens and Antibiotic-Resistant Bacteria. J. Mar. Sci. Eng. 2025, 13, 448. [Google Scholar] [CrossRef]
- Vishnupriya, S.; Jabir, T.; Akhil, P.E.; Mohamed, H.A.A. Antibiotic resistance of heterotrophic bacteria from the sediments of adjoining high Arctic fjords, Svalbard. Braz. J. Microbiol. 2024, 55, 2371–2383. [Google Scholar] [CrossRef]
- Mogrovejo, D.C.; Perini, L.; Gostinčar, C.; Sepčić, K.; Turk, M.; Ambrožič-Avguštin, J.; Brill, F.H.H.; Gunde-Cimerman, N. Prevalence of Antimicrobial Resistance and Hemolytic Phenotypes in Culturable Arctic Bacteria. Front. Microbiol. 2020, 11, 570. [Google Scholar] [CrossRef]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic pollution in the environment: From microbial ecology to public policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef]
- Burgess, A.; Glasauer, P. Why we need to eat well. In Family Nutrition Guide; Burgess, A., Glasauer, P., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- United Nations. Universal Declaration of Human Rights. Un.org. 2024. Available online: https://www.un.org/en/about-us/universal-declaration-of-human-rights#:~:text=Article%203,liberty%20and%20security%20of%20person (accessed on 18 March 2025).
- United Nations. United Nations Charter (full text). Un.org. 2024. Available online: https://www.un.org/en/about-us/un-charter/full-text (accessed on 14 April 2025).
- Gasper, D.; Jolly, R.; Koehler, G.; Kool, T.; Simane, M. Adding Human Security and Human Resilience to Help Advance the SDGs Agenda. ISS Working Paper Series/General Series (Volume 665). 2020. Available online: http://hdl.handle.net/1765/131247 (accessed on 12 March 2025).
- Fogarty, I. The World Heritage Convention, human rights and Indigenous peoples: A critical review. Hunt. Gatherer Res. 2024, 1–26. [Google Scholar] [CrossRef]
- Gómez, J.F.M. Rethinking the human right to food from a single perspective to a four-fold legal interpretation. J. Hum. Rights Pract. 2024, 16, 589–602. [Google Scholar] [CrossRef]
- Lougheed, T. The changing landscape of arctic traditional food. Environ. Health Perspect. 2010, 118, A386–A393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meltofte, H.; Barry, T.; Berteaux, D.; Bültmann, H.; Christiansen, J.S.; Cook, J.A.; Dahlberg, A.; Daniëls, F.J.A.; Ehrich, D.; Fjeldså, J.; et al. Synthesis: Implications for Conservation. In Arctic Biodiversity Assessment; Meltofte, H., Josefson, A.B., Payer, D., Eds.; Status and trends in Arctic Biodiversity; Arctic Council: Tromsø, Norway, 2013; pp. 20–65. [Google Scholar]
- Muir, D.; Gunnarsdóttir, M.J.; Koziol, K.; von Hippel, F.A.; Szumińska, D.; Ademollo, N.; Corsolini, S.; De Silva, A.; Gabrielsen, G.; Kallenborn, R.; et al. Local sources versus long-range transport of organic contaminants in the Arctic: Future developments related to climate change. Environ. Sci. Adv. 2025, 4, 355–408. [Google Scholar] [CrossRef]
- Miner, K.R.; Blais, J.; Bogdal, C.; Villa, S.; Schwikowski, M.; Pavlova, P.; Steinlin, C.; Gerbi, C.; Kreutz, K.J. Legacy organochlorine pollutants in glacial watersheds: A review. Environ. Sci. Process. Impacts 2017, 19, 1474–1483. [Google Scholar] [CrossRef]
- Spataro, F.; Patrolecco, L.; Ademollo, N.; Præbel, K.; Rauseo, J.; Pescatore, T.; Corsolini, S. Multiple exposure of the Boreogadus saida from bessel fjord (NE Greenland) to legacy and emerging pollutants. Chemosphere 2021, 279, 130477. [Google Scholar] [CrossRef]
- Gilbert, S.Z.; Walsh, D.E.; Levy, S.N.; Maksagak, B.; Milton, M.I.; Ford, J.D.; Hawley, N.L.; Dubrow, R. Determinants, effects, and coping strategies for low-yield periods of harvest: A qualitative study in two communities in Nunavut, Canada. Food Secur. 2021, 13, 157–179. [Google Scholar] [CrossRef]
- Wang, S. Opportunities and threats of cryosphere change to the achievement of UN 2030 SDGs. Humanit. Soc. Sci. Commun. 2024, 11, 1–13. [Google Scholar] [CrossRef]
- Christensen, K. Thawing Permafrost Releases Industrial Contaminants into Arctic Communities. Environ. Health Perspect. 2024, 132, 32001. [Google Scholar] [CrossRef]
- Mustonen, T.; Ford, V. Indigenous peoples and biodiversity in the Arctic. In Arctic Biodiversity Assessment; Meltofte, H., Josefson, A.B., Payer, D., Eds.; Status and trends in Arctic biodiversity; Arctic Council: Tromsø, Norway, 2013; pp. 18–19. [Google Scholar]
- Rautio, A.; Poppel, B.; Young, K. Human Health and Well-Being. In Arctic Human Development Report; Larsen, J.N., Fondahl, G., Eds.; Regional Processes and Global Linkages; Norden: Hellerup, Denmark, 2014; pp. 299–348. [Google Scholar]
- American Psychological Association. APA Dictionary of Psychology. Apa.org. 2024. Available online: https://dictionary.apa.org/resilience (accessed on 15 March 2025).
- Arctic Council. Summary for policy-makers. In Arctic Resilience Interim Report 2013; Arctic Council: Tromsø, Norway, 2013. [Google Scholar]
- Bölter, M.; Müller, F. Resilience in polar ecosystems: From drivers to impacts and changes. Polar Sci. 2016, 10, 52–59. [Google Scholar] [CrossRef]
- Larsen, J.N.; Anisimov, O.A.; Constable, A.; Hollowed, A.B.; Maynard, N.; Prestrud, P.; Prowse, T.D.; Stone, J.M.R. Polar regions. In Climate Change 2014: Impacts, Adaptation and Vulnerability: Part B: Regional Aspects: Working Group II Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press & Assessment: Cambridge, UK, 2014; pp. 1567–1612. [Google Scholar]
- Kubiszewski, I.; Adams, V.M.; Baird, R.; Boothroyd, A.; Costanza, R.; MacDonald, D.H.; Finau, G.; Fulton, E.A.; King, C.K.; King, M.A.; et al. Cascading tipping points of Antarctica and the Southern Ocean. AMBIO 2024, 54, 642–659. [Google Scholar] [CrossRef] [PubMed]
- Potapowicz, J.; Szopińska, M.; Szumińska, D.; Bialik, R.J.; Polkowska, Ż. Sources and composition of chemical pollution in Maritime Antarctica (King George Island), part 1: Sediment and water analysis for PAH sources evaluation in the vicinity of Arctowski station. Chemosphere 2021, 288, 132637. [Google Scholar] [CrossRef] [PubMed]
- Szopińska, M.; Szumińska, D.; Bialik, R.J.; Dymerski, T.; Rosenberg, E.; Polkowska, Ż. Determination of polycyclic aromatic hydrocarbons (PAHs) and other organic pollutants in freshwaters on the western shore of Admiralty Bay (King George Island, Maritime Antarctica). Environ. Sci. Pollut. Res. 2019, 26, 18143–18161. [Google Scholar] [CrossRef] [PubMed]
| Foodstuff | POPs | Reference |
|---|---|---|
| Egg | Dioxins/furans, PCBs, OCPs, PFCs and HBCDs | [18,19,20,21,22,23,24] |
| Dairy product (milk, butter, cheese, cream, yogurt, ice cream, etc.) | Dioxins/furans, PCBs, OCPs and PAHs | [18,19,23,25,26,27,28] |
| Meat and meat product (pork, chicken, beef, sausage, etc.) | Dioxins/furans, PCBs, OCPs, HCBD and PCN | [18,20,21,23,24,29,30] |
| Grain, flour, and bran | PAHs | [28] |
| Rice, fruit, and vegetable (cabbage, carrot, potato, etc.) | OCPs, PCBs and PAHs | [27,31,32,33] |
| Honey | OCPs | [28,34] |
| Oil (vegetable oil, olive oil, etc.) | Dioxins/furans, PCBs, OCPs and HBCDs | [20,29,35] |
| Fish | OCPs, PCBs, PBDEs, PFOS, Dioxins/furans and HBCDs | [19,21,22,23,26,36,37,38,39,40,41,42] |
| Mussel | OCPs, PCBs and PBDEs | [40,43,44] |
| Oyster | PAHs | [28] |
| Water | PFOS, OCPs, PCBs and PAHs | [31,40] |
| POP | Detection Method | Health Hazards | Tolerable Daily Intake | References |
|---|---|---|---|---|
| PCB | Dispersive liquid–liquid microextraction, Solid-phase extraction, Gas chromatography-Mass Spectrometry (GC–MS), Atmospheric pressure gas chromatography (APGC) | Cancer (breast, prostate, testicular, kidney, ovarian and uterine cancers), neurological disorders, endocrine disruption, liver injury, diabetes, cardiovascular problems and obesity | 1–4 pg TEQ kg−1; 2 mg/kg ww. | [21,54,55,56] |
| PBDEs | Dispersive liquid–liquid microextraction, Solid-phase extraction, GC–MS | Diabetes, obesity and cardiovascular problems, reproductive problems, cancer (testicular) | None | [54,57] |
| OCPs | Dispersive liquid–liquid microextraction, Solid-phase extraction | Neurological symptoms, diabetes, cancer (breast, testicular, prostate and kidney cancer), endocrine disruption, infertility and fetal malformation, reproductive issues, cardiovascular issues, high blood pressure, glucose intolerance and obesity | varying | [21,54,58,59,60] |
| PAHs | Dispersive liquid–liquid microextraction | Mutagenicity and carcinogenicity, DNA damage, cognitive dysfunction among children and cancer (breast cancer), oxidative stress, impaired male fertility, respiratory diseases, | 2–20 micrograms | [21,28,54,61] |
| PFOS | Solid-phase extraction | Breast cancer | immune toxicity at 0.63 ng/kg body weight | [54,62] |
| PFOA | Solid-phase extraction | Breast cancer | 0.0000015–0.16 μg/kg-day | [54,63] |
| PCDD/Fs | GC coupled with a high-resolution MS | Thyroid hormone endocrine balance of infants and children and their mothers, infectious diseases | 1–4 pg TEQ kg−1 | |
| HBCD | Liquid chromatography (LC-MS/MS) | Endocrine disruption, reproductive issues, and behavioral effects | 1.2–20 ng/day | [64,65] |
| PCN | GC electron capture detector or GC-MC | Cancers | no-observed-adverse effect level −0.03 mg/kg bw/day | [21,66] |
| PCDE | Isotope dilution GC–MS | Cancers | n.a | [21] |
| Dioxins/Furans | High-resolution MS | Affects motor and mental development, speech delay, cancer, diabetes, endocrine disruption, high blood pressure, glucose intolerance and cardiovascular problems | 1–4 pg TEQ/kg bw/day | [21,55,67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raheem, D.; Trovò, M.; Carmona Mora, C.; Vassent, C. Persistent Organic Pollutants’ Threats and Impacts on Food Safety in the Polar Regions—A Concise Review. Pollutants 2025, 5, 14. https://doi.org/10.3390/pollutants5020014
Raheem D, Trovò M, Carmona Mora C, Vassent C. Persistent Organic Pollutants’ Threats and Impacts on Food Safety in the Polar Regions—A Concise Review. Pollutants. 2025; 5(2):14. https://doi.org/10.3390/pollutants5020014
Chicago/Turabian StyleRaheem, Dele, Marco Trovò, Constanza Carmona Mora, and Clara Vassent. 2025. "Persistent Organic Pollutants’ Threats and Impacts on Food Safety in the Polar Regions—A Concise Review" Pollutants 5, no. 2: 14. https://doi.org/10.3390/pollutants5020014
APA StyleRaheem, D., Trovò, M., Carmona Mora, C., & Vassent, C. (2025). Persistent Organic Pollutants’ Threats and Impacts on Food Safety in the Polar Regions—A Concise Review. Pollutants, 5(2), 14. https://doi.org/10.3390/pollutants5020014







