Abstract
The use of ultraviolet (UV) for water disinfection is known for its chemical-free process and with no harmful disinfection by-products. Yet, the disinfection process remains time-consuming, and many studies are limited to disinfection of one or two microbial species. Direct photolytic and glass-embedded TiO2 photocatalytic disinfection of four different bacterial species (Staphylococcus aureus, Salmonella senftenberg, Bacillus subtilis, and Escherichia coli) were assessed using UV-LED radiation with wavelengths of 365 nm. The optimization of the UV disinfection under different masses of the TiO2 photocatalyst was evaluated. Additionally, the order of disinfection of the different bacteria species was assessed. The disinfection effects were measured based on the potential to reduce the number of bacteria species, calculated in colony-forming units/mL and log reduction units. The disinfection of Staphylococcus aureus was enhanced from 1.46 log reduction units in the UV-alone treatment to a high of 5.65 log reduction units in the UV + 0.08 g TiO2 treatment. Regarding Salmonella senftenberg, disinfection was enhanced from 1.26 log reduction units to 3.85 log reduction units in UV-alone experimental treatments and UV + 0.04 g TiO2, respectively. Similarly, an increase in Bacillus subtilis reduction was achieved from a low of 0.69 log reduction units to a high of 2.98 log reduction units in UV-alone treatments and UV + 0.08 g TiO2, respectively. The disinfection of Escherichia coli was enhanced from 2.49 log reduction units (UV-alone treatment) to a high of 6.35 log reduction units (UV + 0.02 g TiO2). The findings provide key implications and new insights into the studied bacteria species and the future application of porous glass-embedded TiO2 photocatalysts to enhance bacteria disinfection using UV light for improved water.
1. Introduction
Water treatment technologies play an increasingly important role in the efforts to meet the demand for improved drinking water globally. With organic, inorganic, and microbes as the major contaminants for many drinking water sources, especially in low- and middle-income countries, disinfection is central to the advancement of water treatment processes [1,2,3]. Disinfection is a crucial step in the water treatment process that prevents or reduces the risk of waterborne illnesses and ensures the safety of drinking water [4]. Several disinfection techniques including chloramination, pasteurization, chlorination, chlorine dioxide, and ozonation are used in the treatment of water. But due to concerns over cost-effectiveness, overdose of chemicals, disinfection by-products, and health, there is a shift towards solar water disinfection [5,6]. According to studies, ultraviolet (UV) is an efficient method of water disinfection. UV radiation exhibits effective disinfection capacity against a variety of pathogenic bacteria and has been widely used throughout the world [3,7]. Unlike chemical disinfection, UV radiation also functions as a chemical-free process with a broad-spectrum antimicrobial agent and no risk of overdosing [4,8,9]. Solar water disinfection systems (SODISs) that solely depend on UV radiation from the sun as argued by Afitiri et al. [10] are therefore the most suitable alternative water treatment technologies for populations lacking improved water systems and reliable energy.
However, many pathogenic micro-organisms as reported in several studies, evolve to develop efficient deoxyribonucleic acid (DNA) repair mechanisms in order to counteract the lethal effects of UV-induced damages [11]. This according to studies often occurs either through photoreactivation or dark repair [12,13]. In photoreactivation, the photolyase enzyme specifically binds to the cyclobutane pyrimidine dimers (CPDs) and reverses the damage using the energy of light within the range of 310–480 nm. Dark repair on the other hand replaces the damaged DNA with new, undamaged nucleotides as the efficiency of the UV irradiation reduces or becomes totally unavailable [14,15]. These microbial evolution processes and the development of resistance increasingly challenge UV as the only disinfection method. Moreover, its capacity for scaling to meet the growing needs of larger populations is limited by its characteristically slower disinfection rates. Recent evidence also shows the risk of bacteria regrowth during post-treatment water storage and the quality of the water to be treated [16,17,18,19]. Optimizing UV to address these emerging challenges is now of growing importance.
The integration of photocatalysts into conventional SODIS systems can increase both the disinfection efficiency and organics degradation to improve the quality of the water [10,20]. Photocatalysts integration into conventional SODIS significantly influences the required time for complete disinfection of contaminants in relation to UV alone, non-recovery of inactivated organisms, as well as the inactivation of UV-resistant micro-organisms [10,21,22,23]. Studies in this respect argue for the availability of suitable catalysts (reagents), sunlight availability and intensity as well as simple operational designs to effectively enhance photocatalyst adoption in UV disinfection processes. Hence, more research tailored towards this scope is novel and imperative.
One of the most widely studied photocatalysts for water disinfection has been TiO2; hence, the literature extensively affirms its application to completely disinfect microbial contaminants to increase water access [24,25,26]. When the TiO2 in water is exposed to light energy equal to or greater than its band gap (λ < 385 nm), it induces the generation of holes (h+) and hydroxyl (OH•) in the valence band and electrons (e−) and superoxide ions (•O2−) in the conduction band [12]. As OH• and •O2− radicals are produced on the surface of TiO2, they immediately interact with the organism’s outer surface until they penetrate into its cells [27,28,29]. Compared to the •O2− that are long-lived and cannot penetrate the cell membrane, the OH• radicals are short-lived, highly toxic towards micro-organisms, and capable of oxidizing organic compounds and their diffusion from the TiO2 surface into the solution is minimal. Hence, OH• radicals are the key species in the photocatalytic oxidation process [12,30,31].
The reported remarkable achievement in the use of photocatalysts such as TiO2 in solar water disinfection is a result of the huddle effect provided by the photon energy from the UV light and the chemical energy from the OH• radicals generated from the surface of the UV irradiated TiO2. The UV light inhibits the normal replication of DNA and also bacterial mutations. The cumulative effect of the UV and the OH• radicals becomes highly toxic towards micro-organisms leading to the perturbation of different cellular processes and finally to the death of microbial species [12,32]. In the disinfection of microbial species, UV of a suitable wavelength is needed to match the energy gap of the photoactive TiO2 for the huddle effect to occur [10,33].
Laboratory demonstrations provide a first-hand opportunity to investigate suitable catalysts and UV intensity and radiation on the disinfection of pathogenic bacterial species in unimproved drinking water sources. The most commonly used artificial sources of UV light for laboratory-scale studies are conventional mercury, low-pressure (LP) and medium-pressure (MP) UV lamps, and broadband UV-A lamps. These lamps, however, are disadvantaged as they are characterized by fixed wavelength, short bulb lifetime, low energy efficiency, and environmental pollution due to mercury [12,34].
In the past decade, a new UV source called an ultraviolet light-emitting diode (UV-LED) emerged and has shown the potential to replace conventional UV mercury lamps in water disinfection [4,7,35]. UV-LED applications for water disinfection are characterized by different wavelengths whose emission wavelengths are approximately 300–400 nm (near-ultraviolet light-emitting diodes) and approximately 200–300 nm (deep-ultraviolet LEDs) [12,36]. Additional advantages of UV-LEDs in water disinfection over mercury lamps include no disposal problem (LEDs do not contain mercury), compact and robust design, more durability in transit and during handling, faster start-up time (no warm-up time), ability to turn on and off with high frequencies, lower voltages, low power requirements, potential for higher energy efficiency, potential for longer lifetimes, and reduced frequency of replacement among others [37,38,39].
Li et al. [4] evaluated the comparison of UV-LED and low-pressure UV for water disinfection. Photoreactivation and dark repair of Escherichia coli (E. coli) revealed the distinct roles of different UV lights in the disinfection and reactivation of E. coli. Li et al. [4] concluded that, based on the output power of 280 nm LEDs (two times more than that of 265 nm LEDs) and the reactivation performance, 280 nm LEDs were a better choice for inactivating E. coli. However, studies on more micro-organisms and 365 nm wavelengths are nascent. Similarly, few studies in the literature have evaluated the use of UV-LED/TiO2 disinfection systems for different microbial species in water. Studies in these regards are necessary for a more comprehensive understanding of UV disinfection based on LEDs and adoption in rural settings that still depend on contaminated water sources.
The use of UV for drinking water disinfection is known for no harmful disinfection by-products. Yet, the disinfection process remains time-consuming and limited to the disinfection of one or two microbial species for most studies in the literature. In this study, the disinfection of four different bacteria species (Salmonella senftenberg (S. senftenberg), Bacillus subtilis (B. subtilis), Staphylococcus aureus (S. aureus) and E. coli using UV under laboratory conditions (UV-LED of 365 nm wavelength) was evaluated and the UV disinfection process optimized using photocatalyst (TiO2). The increasing order of disinfection between the studied microbial contaminants was determined. Additionally, the cumulative effects of UV and different masses (0.02 g, 0.04 g, 0.08 g, and 0.16 g TiO2) of the photocatalyst were assessed and compared to inform future applications in field scale demonstrations for improved water access for households.
2. Materials and Methods
2.1. Sample Preparation
We used S. senftenberg, B.subtilis, S. aureus and E. coli as the test micro-organisms in these experiments. A volume of 200 mL nutrient broth II (NB II) was prepared by weighing 2.6 g of NB II into a 500 mL flask and autoclaving it at 121 °C for 15 min. The NB II was then cooled and stored in the refrigerator for further use.
All bacteria species were from the Chair of Biotechnology of Water Treatment, Brandenburg University of Technology, Cottbus-Senftenberg. Each bacteria species was generated from frozen stocks by streaking onto an agar plate (one to two loops into a prepared nutrient broth II (NB II)) and incubated at 37 °C for 18–24 h (h). Single colonies were then inoculated in two aliquots of 5 mL sterile test tube with prepared nutrient broth II (NB II) and then incubated at 37 °C for 18–24 h. The bacteria suspensions were washed 3 times after centrifuging at 5 revolutions per minute (rpm) for 5 min (min) in 6 Eppendorf tubes (2 mL). The bacterial pellets were re-suspended in 12 mL of deionized water and centrifuged (5 rpm, 5 min). The final pellets were then suspended in deionized water in Eppendorf tubes and a 10-fold serial dilution was prepared into sterile test tubes. The initial concentration of the bacteria (CFU/mL (colony-forming units per milliliter) was determined using a spectrophotometer ((BECKMAN, DU 640 made in the Brea, CA, USA). An initial bacteria concentration range of 106–107 CFU/mL was finally prepared into the reactor.
2.2. Experimentation
2.2.1. UV Disinfection Procedure
The experiments were carried out from 1 December 2022 to 18 May 2023. All experiments were carried out under controlled environmental conditions of temperature 25 °C. Additionally, all glassware used in the experimental procedures was washed with distilled water and thermally sterilized at 150 °C for 8 h. The experiments were performed in Petri dishes as simple reactors. Table 1 shows the experimental treatments used in this study. Seven experimental set-ups with two parallels were designed. The experiments included a control experiment in the dark where bacteria species were not exposed to UV-LED irradiation and without the porous glass TiO2 (hereafter treatment 6). The last treatment was TiO2 (0.08 g) in bacteria suspension in the dark (hereafter treatment 7). Another experimental treatment was the prepared sample exposed to UV-LED irradiation without any catalyst (hereafter treatment 1). The other treatments included treatments with 0.02 g porous glass TiO2 exposed under UV-LED radiation (hereafter treatment 2), 0.04 g porous glass TiO2 exposed under UV-LED radiation (hereafter treatment 3), 0.08 g porous glass TiO2 exposed under UV-LED radiation (hereafter treatment 4), and 0.16 g porous glass TiO2 exposed under UV-LED radiation (hereafter treatment 5).

Table 1.
Description of experimental treatments.
The reaction suspension was illuminated with UV-LED radiation for a maximum of 360 min. The distance between the reactor and the light source was fixed at a height of 30 cm. The power irradiance from the UV-LED light was 1500 uW/cm2. A voltage of 5 V, UV grade of 6, and UVI index of 15 were used throughout the experiments.
Regarding the evaluation of the number of micro-organisms, the standard plate count technique was used. The method is one of the most frequently applied in microbiological research and can provide valuable and highly relevant information. 1 mL of the suspension was collected every 30 min. The next serial fourfold dilutions were appropriately performed. 0.01 mL of diluted suspension was dropped over the surface of the NA plate and then incubated at 37 °C for 18–24 h. After incubation, the number of viable colonies was counted and calculated in log reduction units and CFU/mL. The control experiments in the dark were also performed. All experiments were repeated 5 times to obtain reliable results. These procedures were adopted for all the bacteria species in this study.
2.2.2. Porous Glass Imbedded TiO2
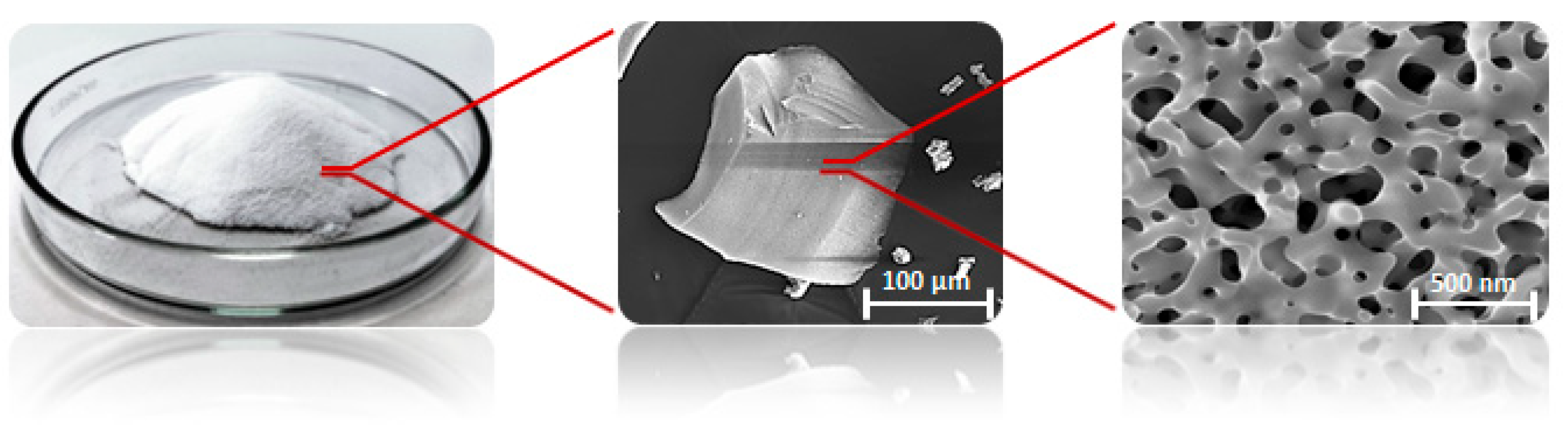
The experiments were performed with different masses of the porous glass-embedded with TiO2 (TiO2 photocatalyst). The titanium-doped glass (BR010) was developed and provided by FEW Chemicals GmbH, Germany. The structure of the microporous materials is shown in Figure 1.
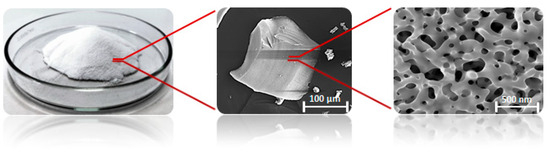
Figure 1.
MAKROSPEC® glass granulate in different magnifications [40].
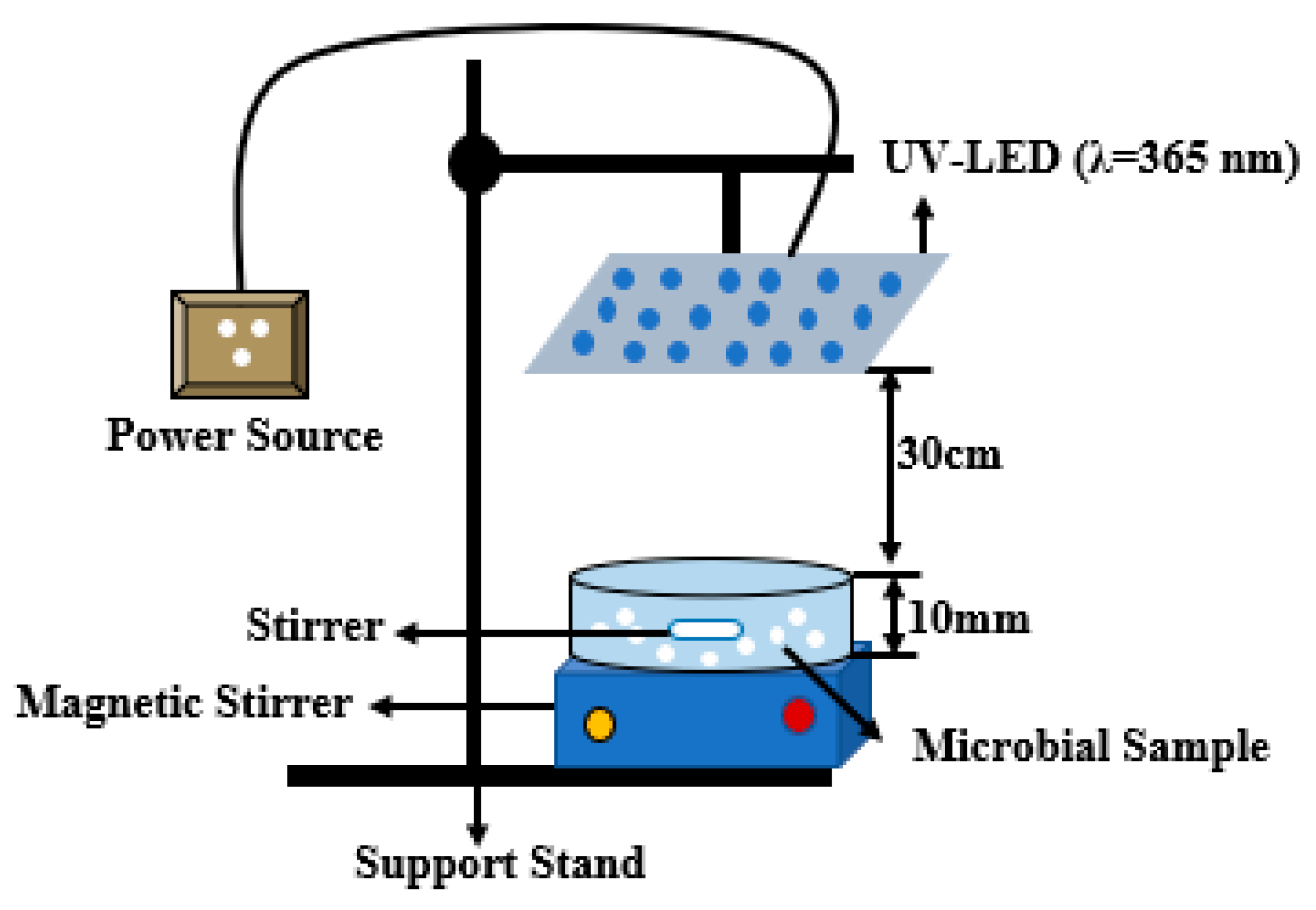
2.2.3. Reactor Configuration and Experimental Set-Up
The UV-LED reactors as shown in Figure 2 are glass Petri dishes. All experiments were carried out under controlled conditions in the laboratory using UV-LED irradiation at a wavelength of 365 nm. An ultraviolet radiation monitor (model KF-90, voltage 5 V), which measures the UVI index, UV grade, and UV radiation, was used. A UVI index of 15, UV grade of 6, and UV radiation of 1500 uW/cm2 was used throughout the experiment. The Petri dishes were borosilicate glass (DURAN, Schott, Germany). A borosilicate glass rod (DURAN, Schott, Germany) was first used to stir the sample on a tray at 137 rpm before taking 20 mL of the sample into the Petri dishes.
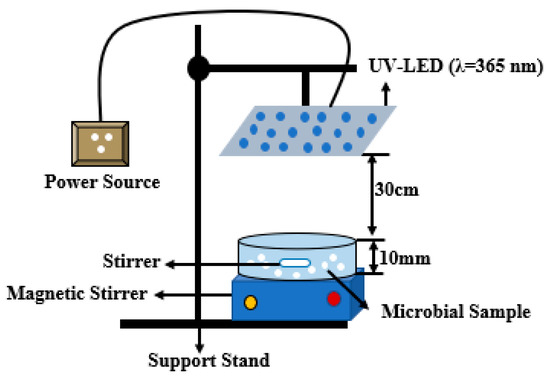
Figure 2.
Reactor configuration for the experiment.
Prior to the exposure to the UV-LED irradiation, 200 mL of deionized water was measured into Borosilicate bottles and spiked with bacteria to a desired concentration range of 106–107 CFU/mL solution. The reaction suspension was continuously stirred on a shaker for 10 min at 137 rpm to ensure homogeneity. Photocatalysts (porous glass TiO2) were measured in different masses of 0.02 g, 0.04 g, 0.08 g, and 0.16 g into the Petri dishes and 20 mL of the suspension pipetted into the Petri dishes before exposure to the UV irradiation.
2.2.4. Data and Statistical Analyses
The disinfection effects of UV and TiO2 were measured based on the potential to reduce the number of the bacterium for each bacteria species calculated in CFU/mL and log reduction units. For analytical purposes, the various experimental treatments in this study were recorded differently (Table 1): UV alone as 1; UV + 0.02 g TiO2 as 2; UV + 0.04 g TiO2 as 3; UV + 0.08 g TiO2 as 4; and UV + 0.16 g TiO2 as 5. A one-way analysis of variance (ANOVA) was then implemented to evaluate whether the means of the disinfected bacterium differed statistically with respect to the experimental treatments (i.e., for each of the four bacteria species). We further conducted a post hoc test (Tukey’s test) to identify which specific experimental treatment accounted for observed differences as well as inform the best experimental treatment for the disinfection of a particular bacteria species. A statistical significance level (P value) set at 0.05 with a 95% confidence interval (CI) was used. All statistical analyses were performed in Stata 15 (StataCorp, College Station, TX, USA) SE software.
3. Results
3.1. Dark Experiments
The two experimental set-ups that were kept in the dark with and without TiO2 for the four bacteria species (S. aureus, S. senftenberg, B. subtilis, and E. coli) (treatments 6 and 7) showed no inactivation after 6 h. This therefore signified that disinfection under dark conditions with or without a photocatalyst does not occur. On the other hand, the concentration of the treatments informs the viability of the four bacteria species for the experiments.
3.2. Disinfection of S. aureus with UV-LED Radiation and Different Masses of Porous Glass TiO2
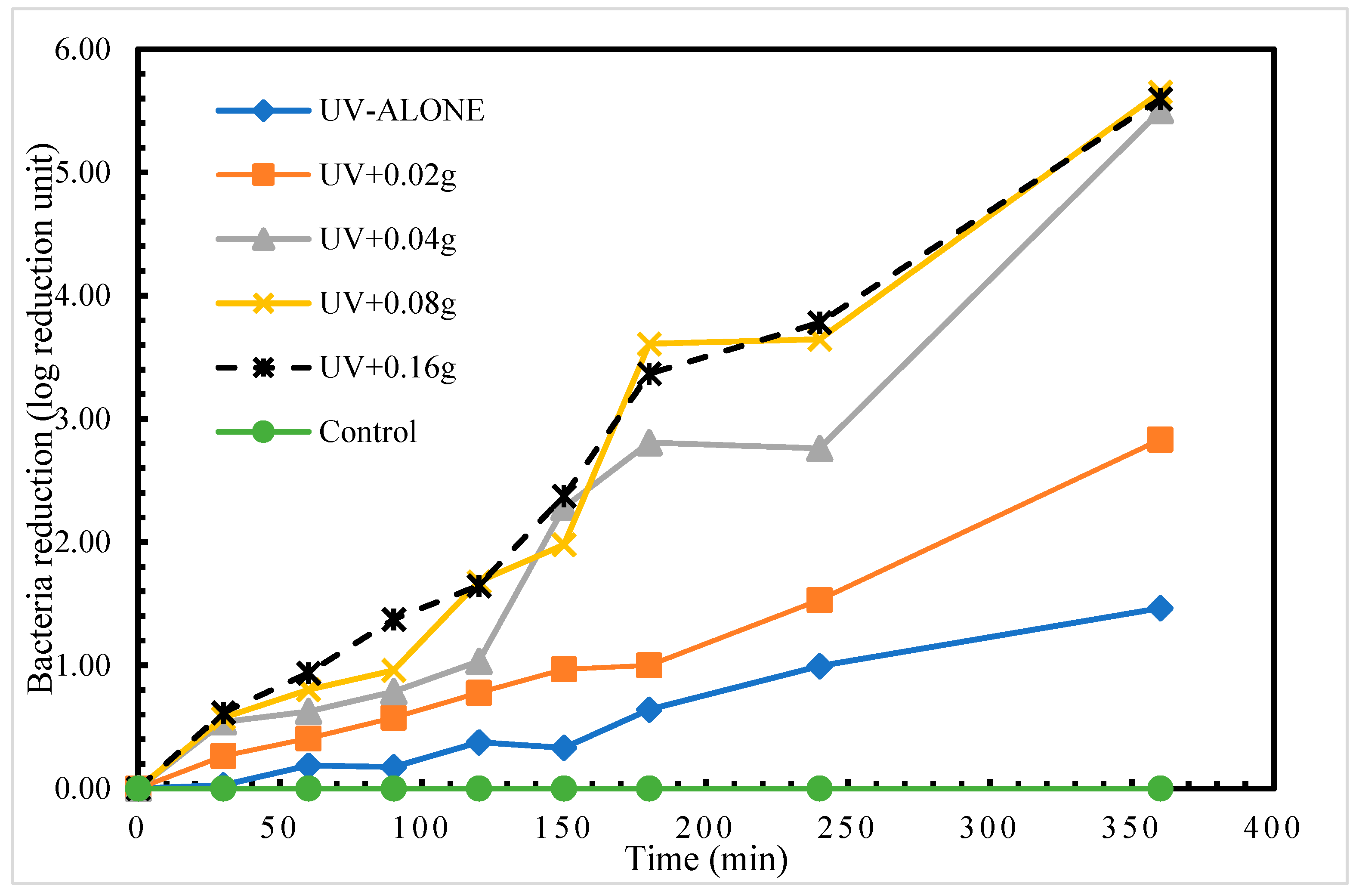
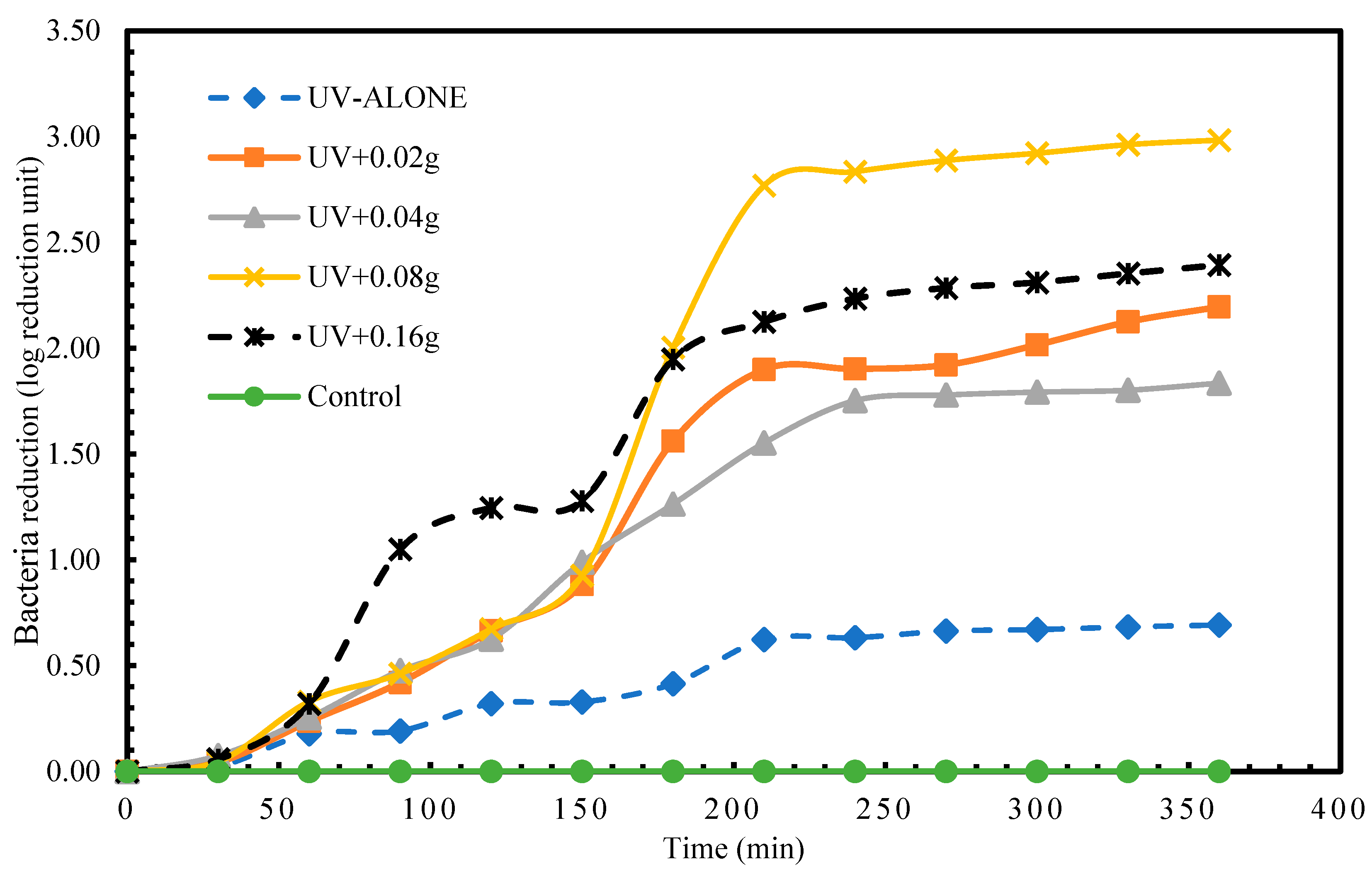
We found disinfection of S. aureus in treatment with UV radiation alone. The highest log reduction of 1.46 log reduction units was achieved at time 360 min. This therefore indicates that using UV radiation alone to disinfect S. aureus required a longer exposure time to achieve a higher log reduction (complete disinfection). Comparatively, the application of different masses of the porous glass TiO2 gave very intriguing results. The highest recorded disinfection of 5.65 log reduction units at time 360 min was measured in treatment UV + 0.08 g TiO2 as shown in Figure 3. After 24 h post-treatment storage, a regrow of S. aureus was detected in only treatment 1 (UV alone).
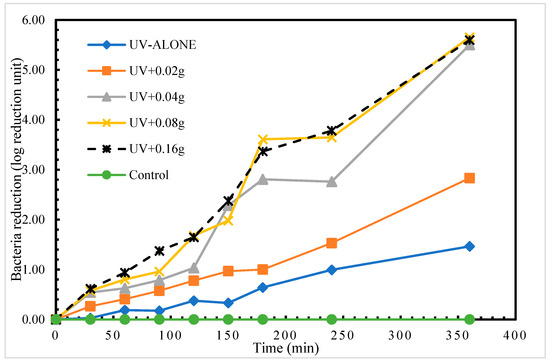
Figure 3.
Log reduction in S. aureus depending on mass of TiO2.
One-way analysis of variance (ANOVA) test shows statistically significant (p < 0.05) differences in the means of the disinfection rates for the four treatments (Table 2). A further exploration to identify which specific treatments statistically differed in disinfection rate was conducted using a post hoc test (Tukey’s test). The results as presented in Table 3 showed a significant pairwise difference between all the treatments with TiO2 and the UV-alone treatment (p < 0.05). This implies that any mass of TiO2 (0.02 g, 0.04 g, 0.08 g, or 0.16 g) significantly optimizes S. aureus disinfection with UV-LED radiation alone. However, no statistically significant difference was observed between the treatments with UV and TiO2.

Table 2.
Results of ANOVA showing differences in means of disinfection for S. aureus.

Table 3.
Results of Tukey’s pairwise comparisons of means with equal variances for S. aureus.
3.3. Disinfection of S. senftenberg with UV-LED Radiation and Different Masses of Porous Glass TiO2
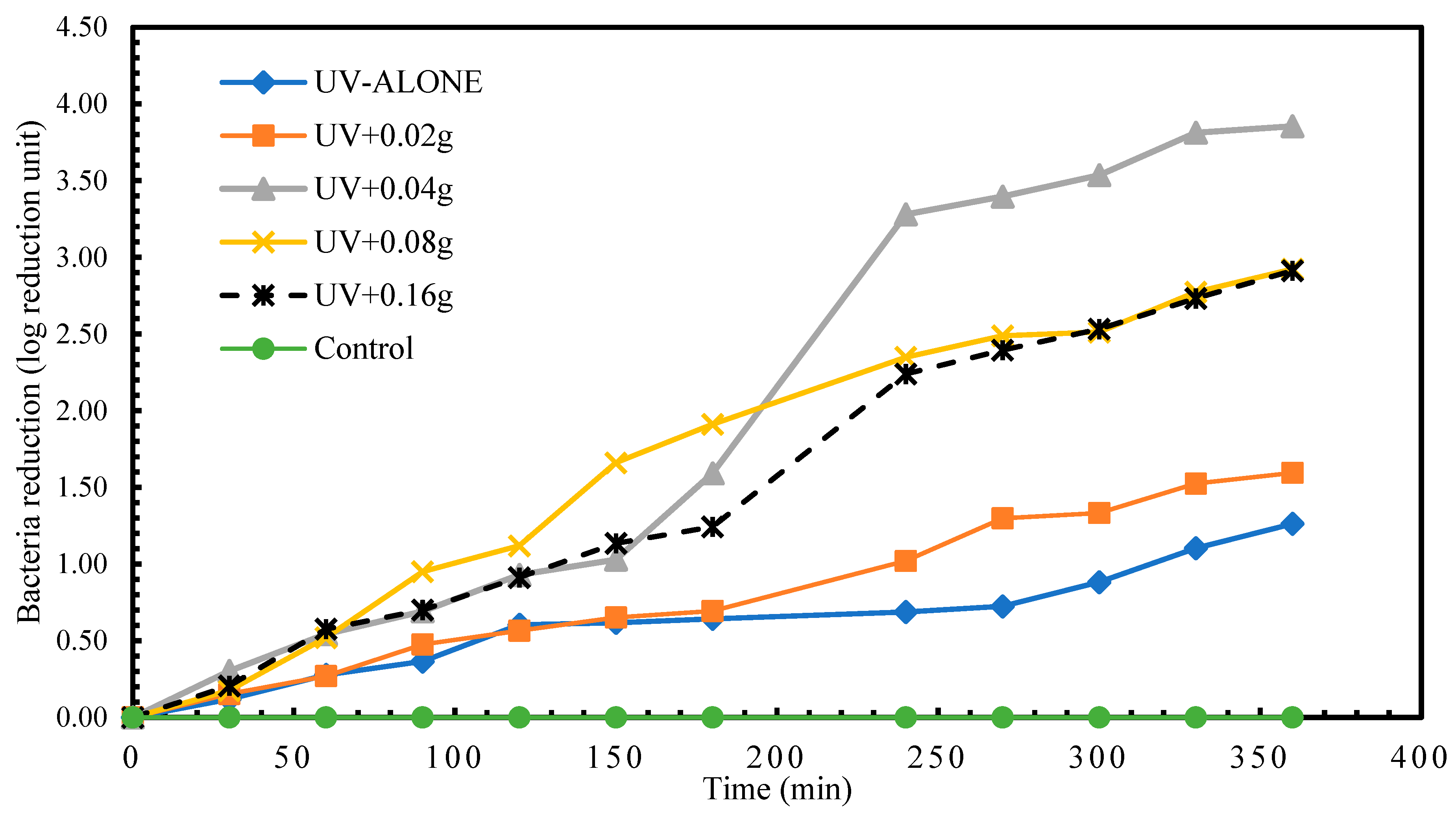
Results for the disinfection of S. senftenberg showed that the highest disinfection achieved in treatment with UV radiation alone was 1.26 log reduction units at time 360 min. A reduction in S. senftenberg of 1.26 log reduction units was already attained in less than 150 min in experimental treatment UV + 0.08 g TiO2. The highest S. senftenberg reduction (3.85 log reduction units) was achieved in treatment UV+0.04g TiO2 treatment at time 360 min (Figure 4). Experimental treatment 1 recorded a regrow of S. senftenberg after 24 h post-treatment storage.
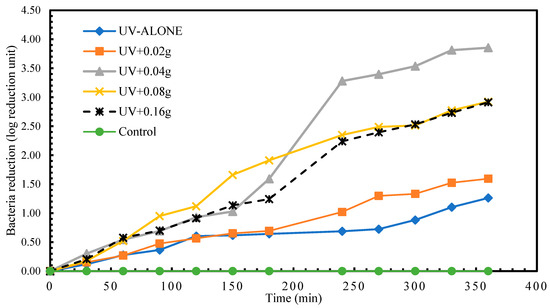
Figure 4.
Log reduction in S. senftenberg depending on mass of TiO2.
As shown in Table 4 there is a statistically significant difference (p < 0.05) in the means of the different experimental treatments for S. senftenberg disinfection. Tukey’s test (Table 5) revealed that this difference is between experimental treatment 3 and 1 (UV + 0.04 g TiO2 and UV alone) (p < 0.05) as well as 4 and 1 (UV + 0.08 g TiO2 and UV alone) (p < 0.05). No statistically significant differences were observed between the rest of the compared experimental treatment groups.

Table 4.
Results of ANOVA showing differences in means of disinfection for S. senftenberg.

Table 5.
Results of Tukey’s pairwise comparisons of means with equal variances for S. senftenberg.
3.4. Disinfection of B. subtilis with UV-LED and Different Masses of Porous Glass TiO2
In the disinfection of B. subtilis, the highest reduction at time 360 min. for experimental treatment 1 (UV alone) was 0.69 log reduction unit. Within 90 min. of the experiments, a log reduction unit of 0.69 was already achieved in the treatments with photocatalyst (TiO2). As illustrated in Figure 5, the highest disinfection (2.98 log reduction units) achieved at 360 min. was in experimental treatment 4 (UV + 0.08 g). There existed a statistically significant difference (p < 0.05) between the means of the various experimental groups (Table 6). The Tukey’s test results, as Table 7 shows, indicate that the difference is between treatments 3 vs. 1, 4 vs. 1, 5 vs. 1, 4 vs. 2, and 5 vs. 2. The rest of the pairwise comparisons in the experimental treatments did not reveal statistical significance.
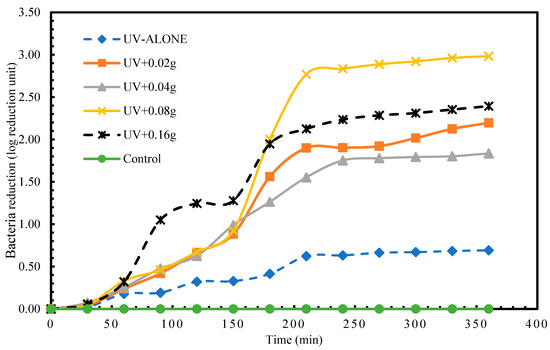
Figure 5.
Log reduction in B. subtilis depending on mass of TiO2.

Table 6.
Results of ANOVA showing differences in means of disinfection for B. subtilis.

Table 7.
Results of Tukey’s pairwise comparisons of means with equal variances for B. subtilis.
3.5. Disinfection of E. coli with UV-LED and Different Masses of Porous Glass TiO2
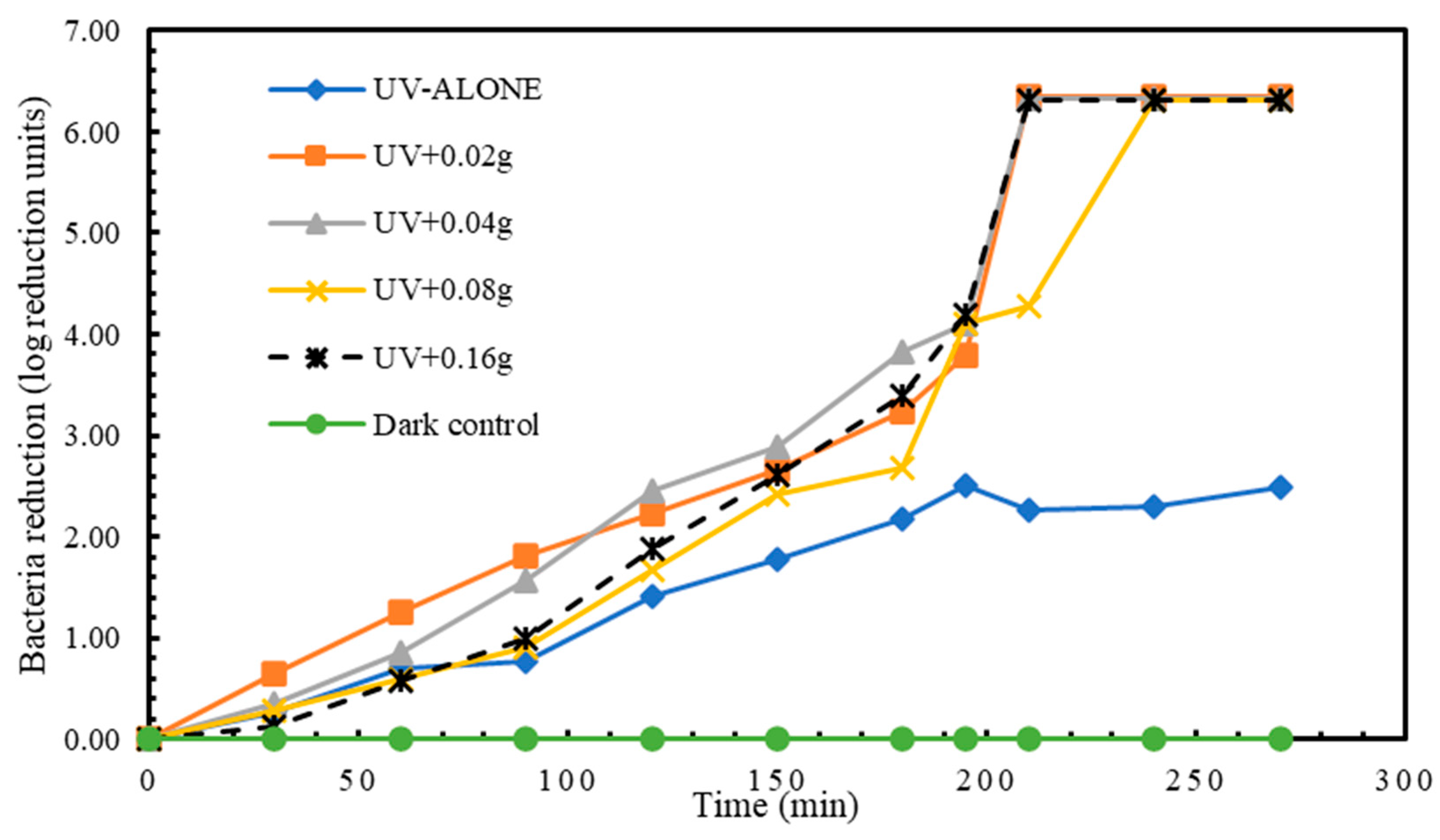
Higher reductions in E. coli were attained compared to the other 3 bacteria species investigated in this study. Treatment 1 (UV alone) had a highest disinfection of 2.49 log reduction units in 270 min. The experimental treatment 2 (UV + 0.02 g TiO2) achieved the highest reduction of 6.35 log reduction units in 270 min. (Figure 6). Even though differences existed in the E. coli reduction means among the experimental treatments, the differences were not statistically significant from each other (Table 8 and Table 9).
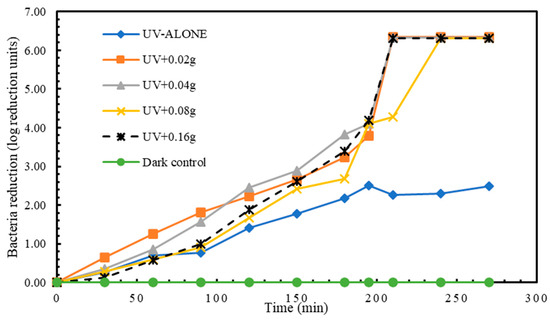
Figure 6.
Log reduction in E. coli depending on mass of TiO2.

Table 8.
Results of ANOVA showing differences in means of disinfection for E. coli.

Table 9.
Results of Tukey’s pairwise comparisons of means with equal variances for E. coli.
3.6. Comparison of the Disinfection of the Four Bacteria Species- S. aureus, S. senftenberg, B. Subtilis, and E. coli
The disinfection data for all the studied bacteria species were compared to assess whether the disinfection rates for the various species vary significantly from each other. The results as presented in Table 10 revealed that the means of the disinfected bacteria species are different. With the exception of the means of S. aureus vs. S. senftenberg, which were not statistically significant, all other pairwise comparisons were statistically significant (Table 11).

Table 10.
Results of ANOVA showing differences in means of disinfection for all four bacteria species.

Table 11.
Results of Tukey’s pairwise comparisons of means with equal variances for all four bacteria species.
4. Discussion
This study evaluated the disinfection of S. aureus, S. senftenberg, B. subtilis, and E. coli using UV-LED (365 nm) and the optimization of the disinfection with different masses of photocatalyst (TiO2). Additionally, the order of disinfection of the different bacteria species was assessed. No bacterial disinfection was observed under dark conditions. The no inactivation of the bacterial species under dark conditions indicates that the photolytic or photocatalytic disinfection observed is exclusively attributed to the UV light derived from UV-LED. We found that porous glass TiO2 photocatalyst alone in the dark had no effect on the disinfection of all the studied microbial organisms. UV radiation drives the disinfection of microbial organisms and its availability with TiO2 in water produces OH• and •O2− radicals for the photocatalytic oxidation process to produce a huddle effect that kills micro-organisms [12,39]. Hence, without photon energy from UV light, photocatalysts alone cannot remarkably disinfect microbial water contaminants.
The UV-LED radiation had disinfection effects on all the studied bacteria species. The results from this study also demonstrated that 365 nm UV-LED alone is not efficient for micro-organisms’ disinfection and hence affirms one of the key findings from Song et al. [7]. These align with findings from previous studies on the use of UV-LED for water disinfection [9,36,38]. Hijnen et al. [41] found that for UV disinfection, DNA or RNA is the key target in a variety of organisms which comes in two major classes of mutagenic DNA lesions induced by UV irradiation—cyclobutane pyrimidine dimers (CPDs) and 6–4 photoproducts (6–4 PPs) [4,13]. The UV light inhibits the normal replication of DNA and also bacterial mutations, hence leading to perturbation of different cellular processes and finally to the death of microbial species [5,7,24].
Disinfection with UVA (315–400 nm) radiation alone is known to be less efficient. Our observations of low disinfection rates with UV alone for all four bacteria species further supported this. However, the integration of UVA radiation with TiO2 photocatalyst efficiently disinfects micro-organisms through the production of reactive oxygen species [42]. As UVA-LEDs are known for their high output power feature, they are desirable for photocatalytic disinfection with TiO2 [7]. For instance, the “residual disinfecting effect”, a phenomenon where E. coli attains further disinfection even after a photocatalytic process, as observed in previous studies, shows this high output in disinfection rates [43]. Such residual disinfection effects are known for the cumulative damage of cellular components by reactive oxygen species or stable oxidants, such as H2O2, which could prevent the reproduction of damaged micro-organisms [7,44,45,46].
The results support the use of the porous glass-embedded TiO2 for efficient water disinfection. The disinfection of the pathogenic micro-organisms (S. aureus, S. senftenberg, B. subtilis, and E. coli.) under UV-LED irradiation was enhanced with the addition of the porous glass TiO2 photocatalyst. The studied micro-organisms when subjected only to UV light recorded very low log reduction and were able to repair after 24 h post-treatment storage. The use of TiO2 in suspension is efficient due to the large surface area of the catalyst available for the reaction. Studies in the literature show that TiO2 photocatalysts significantly influence the required time for complete disinfection of contaminants in relation to UV alone and non-recovery of disinfected microbial organisms [10,16,21,22,23,47], which is further confirmed in this study. The major reactive oxygen species (ROS), hydroxyl radicals, and hydrogen peroxide induce oxidative damage to DNA, proteins, and cell membranes and cause growth delays in micro-organisms [7,13]. Comparatively, this process requires more time than the direct damage produced by UVC (less than 280 nm) radiation [7,48]. Nonetheless, microbial species disinfection with UVA is believed to be irreparable, whereas the damage by UVC is reparable through DNA repair mechanisms [43,49].
The presence of some of the microbial species after 360 min of the experiments indicates their great resistance to stressful conditions, especially B. subtilis. This resistance is reflected at the same time by the ability of these micro-organisms to regrow after post-treatment storage in the dark for 24 h and after being exposed to UV radiation that could affect their stability or rate of reproduction. This indicates the capability of micro-organisms to outlive the UV disinfection process as reported in similar studies [16,50,51]. The experimental treatments without TiO2 recorded a regrowth of bacterial species after 24 h of post-treatment storage. The establishment of the cellular repair mechanism was beyond the scope of this study. Nonetheless, bacteria recover by means of some of the documented cellular repair mechanisms such as photoreactivation, nucleotide excision repair, mutagenic DNA repair, and recombination DNA repair, thus permitting their recovery and the manifestation of the regrowth phenomenon [52].
The maximum log reduction of 5.65, 3.85, 2.98, and 6.35 were obtained with treatments with 0.08 g, 0.04 g, 0.08 g, and 0.02 g of the TiO2, respectively, for the corresponding bacterial species S. aureus, S. senftenberg, B. subtilis, and E. coli. The disinfection in log reduction units increased over the irradiation time. These findings therefore mean that the addition of TiO2 in suspension leads to an increase in the disinfection of the bacterial species within a shorter period of time which can be attributed to the increase in the OH• generated from the surface of the UV irradiated TiO2 [53]. However, as the higher mass of the TiO2 is introduced, a point is reached where disinfection of the bacterial species decreases. This therefore indicates a detrimental effect that can be attributed to either a screening effect of excess TiO2 particles on the surface of the bacterial species [12,54] or the accumulation of OH• radicals which readily dimerize and react to form hydroperoxyl radicals, which does not contribute to the inactivation process due to their less reactivity [31]. Analogous results to these findings were also reported in previous studies [12,29,55,56,57].
E. coli disinfection achieved the highest log reduction among the studied micro-organisms supporting related works in the literature on E. coli disinfection using UV-LEDs of different wavelengths (255, 265, 272, 275, and 280 nm) with a reported log reduction range of 3.4–4.0 log reduction units [48,49,58,59]. The findings from this study with respect to the disinfection of E. coli are similar to the works of Hamamoto et al. [60] and Mori et al. [61], who applied UV radiation of 365 nm for the disinfection of E. coli and obtained higher log reduction of 5.7 and 3.9 log reduction units, respectively. Xiong and Hu [43] in their study established a 3 log reduction unit for E. coli disinfection using TiO2 photocatalytic disinfection with a 365 nm UV-LED. The higher log reduction obtained for E. coli within a relatively shorter time compared to the other studied bacterial species could have been due to the high flow of photons which directly attack the E. coli bacteria and thus preventing its self-defense and auto-repair mechanisms from taking place leading to accelerated disinfection [12,29]. E. coli therefore demonstrates a more sensitive mechanism to the UV and a huddle effect is expected even at low irradiance from the light source.
After 24 h post-treatment storage, no dark repair was detected in all the TiO2 photocatalyst treatments. The repressed photoreactivation and dark repair in TiO2 photocatalysis could be attributed to the severe damage of the DNA by the UV photon due to high UV dose and severe damage of the cell membrane and oxidative attack of intracellular components by the OH• generated from the surface of the UV irradiated TiO2 that could have led to subsequent cell death [12,62].
Statistically significant associations were established between the compared means of the four studied bacteria species (S. aureus, S. senftenberg, B. subtilis, and E. coli). The comparison of micro-organism response to UV-LEDs in this study, as well as the literature works, revealed that some micro-organisms may be sensitive to different UV sources, likely due to the difference in the UV source radiation patterns and the fluence rates. The order of disinfection of the studied bacteria is consistent with previous works by Wang et al. [63] that demonstrated bacterial inactivation in the order of E. coli, S. aureus, and B. subtilis. Disinfection studies on several micro-organisms using UV-LED of the same wavelengths and comparable fluence rates still show considerable discrepancies among the published results [6,7,64].
5. Conclusions
Direct photolytic and TiO2 photocatalytic disinfection of four different bacterial species were assessed using UV-LED of wavelength 365 nm. The optimization of the UV disinfection under different masses of the TiO2 photocatalyst was evaluated. Additionally, the order of disinfection of the different bacteria species was assessed. The findings demonstrated that the application of UV alone was not efficient for micro-organisms’ disinfection; however, the integration of UV and TiO2 photocatalyst optimized the disinfection of S. aureus, S. senftenberg, B. subtilis, and E. coli in water. The TiO2 alone had no effect on the disinfection of the pathogenic bacteria species. It was revealed that UV radiation of wavelength 365 nm for the disinfection of S. aureus, S. senftenberg, B. subtilis, and E. coli achieved 1.46, 1.26, 0.69, and 2.49 log reduction units, respectively. Apparently, the UV irradiation for the disinfection of the studied microbial species required more exposure time of not less than 6 h for efficient disinfection. The optimization with TiO2 photocatalyst gave corresponding disinfections of 5.65, 3.85, 2.98, and 6.35 log reduction units for S. aureus, S. senftenberg, B. subtilis, and E. coli. The disinfection calculated and reported in a log reduction of the bacteria species in increasing order was B. subtilis < S. senftenberg < S. aureus < E. coli. The findings provide key implications and new insights into the studied bacteria species and the future application of porous glass TiO2 to enhance bacteria disinfection using UV light. It is recommended that future studies investigate the stability of the photocatalyst after the degradation processes, the photocatalyst regeneration, the degradation mechanism, the availability, cost-effectiveness, health implications of the use of porous glass TiO2, and possible adoption in water treatment.
Author Contributions
A.-R.A.: Conceptualization, Methodology, Investigation Data curation, Formal analysis, Writing—original draft, Visualization, Editing of the manuscript, Validation. M.M.: Conceptualization, Methodology, Writing—review and editing, Supervision, Resources, Validation. E.K.A.A.: Writing—review and editing, Validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
All data that support the findings of this study are available on request from the corresponding author.
Acknowledgments
The authors acknowledge the support received from Kathrin Krahl and Jörg Böllmann during the laboratory experiments and the recommendations provided. Special thanks also go to Noman Sohail for his support and Iddrisu Amadu for proofreading the manuscript. Abdul-Rahaman Afitiri expresses his gratitude to the Graduate Research School (GRS) of the Brandenburg University of Technology for the financial support for his Ph.D. work.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Lonnen, J.; Kilvington, S.; Kehoe, S.C.; Al-Touati, F.; McGuigan, K.G. Solar and photocatalytic disinfection of protozoan, fungal and bacterial microbes in drinking water. Water Res. 2005, 39, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Karimi, B.; Mazarji, M.; Moghtaderi, H. Cadmium selenide quantum dot-zinc oxide composite: Synthesis, characterization, dye removal ability with UV irradiation, and antibacterial activity as a safe and high-performance photocatalyst. J. Photochem. Photobiol. B Biol. 2018, 188, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Yuan, F.; Xue, M.; Xue, Y.; Xie, Y.; Ou, J.; Luo, Y.; Hong, Z.; Xie, C. A multifunctional and environmentally safe superhydrophobic membrane with superior oil/water separation, photocatalytic degradation and anti-biofouling performance. J. Colloid Interface Sci. 2022, 611, 93–104. [Google Scholar] [CrossRef]
- Li, G.-Q.; Wang, W.-L.; Huo, Z.-Y.; Lu, Y.; Hu, H.-Y. Comparison of UV-LED and low pressure UV for water disinfection: Photoreactivation and dark repair of Escherichia coli. Water Res. 2017, 126, 134–143. [Google Scholar] [CrossRef]
- Aboushi, A.; Hamdan, M.; Abdelhafez, E.; Turk, E.; Ibbini, J.; Abu Shaban, N. Water disinfection by solar energy. Energy Sources Part A-Recovery Util. Environ. Eff. 2021, 43, 2088–2098. [Google Scholar] [CrossRef]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernández-Ibáñez, P.; Polo-López, I.; Sharma, P.K.; Vennard, A.S.M. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef]
- Song, K.; Mohseni, M.; Taghipour, F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: A review. Water Res. 2016, 94, 341–349. [Google Scholar] [CrossRef]
- Kheyrandish, A.; Mohseni, M.; Taghipour, F. Development of a method for the characterization and operation of UV-LED for water treatment. Water Res. 2017, 122, 570–579. [Google Scholar] [CrossRef]
- Mohaghegh Montazeri, M.; Taghipour, F. Developing an optical module for large-scale UV-LED water disinfection reactors by numerical modeling. J. Photochem. Photobiol. A Chem. 2022, 433, 114184. [Google Scholar] [CrossRef]
- Afitiri, A.-R.; Appah Aram, S.; Martienssen, M. Systematic review of the effects of advanced oxidation processes integration with solar water disinfection for improved drinking water production. Waste Manag. Bull. 2024, 1, 52–59. [Google Scholar] [CrossRef]
- Lazarova, V.; Savoye, P.; Janex, M.L.; Blatchley, E.R.; Pommepuy, M. Advanced wastewater disinfection technologies: State of the art and perspectives. Water Sci. Technol. 1999, 40, 203–213. [Google Scholar] [CrossRef]
- Nyangaresi, P.O.; Qin, Y.; Chen, G.; Zhang, B.; Lu, Y.; Shen, L. Comparison of UV-LED photolytic and UV-LED/TiO2 photocatalytic disinfection for Escherichia coli in water. Catal. Today 2019, 335, 200–207. [Google Scholar] [CrossRef]
- Sinha, R.; Häder, D.-P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef]
- Guo, M.; Hu, H.; Bolton, J.R.; El-Din, M.G. Comparison of low- and medium-pressure ultraviolet lamps: Photoreactivation of Escherichia coli and total coliforms in secondary effluents of municipal wastewater treatment plants. Water Res. 2009, 43, 815–821. [Google Scholar] [CrossRef]
- Sommer, R.; Lhotsky, M.; Haider, T.; Cabaj, A. Uv inactivation, liquid-holding recovery, and photoreactivation of Escherichia coli o157 and other pathogenic Escherichia coli strains in water. J. Food Prot. 2000, 63, 1015–1020. [Google Scholar] [CrossRef]
- Gelover, S.; Gómez, L.A.; Reyes, K.; Teresa Leal, M. A practical demonstration of water disinfection using TiO2 films and sunlight. Water Res. 2006, 40, 3274–3280. [Google Scholar] [CrossRef] [PubMed]
- Helali, S.; Polo-López, M.I.; Fernández-Ibáñez, P.; Ohtani, B.; Amano, F.; Malato, S.; Guillard, C. Solar photocatalysis: A green technology for E. coli contaminated water disinfection. Effect of concentration and different types of suspended catalyst. J. Photochem. Photobiol. A Chem. 2014, 276, 31–40. [Google Scholar] [CrossRef]
- Kehoe, S.C.; Joyce, T.M.; Ibrahim, P.; Gillespie, J.B.; Shahar, R.A.; McGuigan, K.G. Effect of agitation, turbidity, aluminium foil reflectors and container volume on the inactivation efficiency of batch-process solar disinfectors. Water Res. 2001, 35, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Rincón, A.-G.; Pulgarin, C. Absence of E. coli regrowth after Fe3+ and TiO2 solar photoassisted disinfection of water in CPC solar photoreactor. Catal. Today 2007, 124, 204–214. [Google Scholar] [CrossRef]
- Zha, Q.; Chen, H.; Yin, Z.; Deng, Y.; Li, Z.; Chen, Y.; Yang, C.; Yang, H.; Luo, Y.; Xue, M. Highly thermally conductive multifunctional graphene-based composite membrane for remarkable passive heat dissipation and robust superhydrophobicity. Appl. Therm. Eng. 2024, 250, 123469. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.; Ho, S.-H. Advanced oxidation processes for water disinfection: Features, mechanisms and prospects. Chem. Eng. J. 2021, 409, 128207. [Google Scholar] [CrossRef]
- Giannakis, S.; Le, T.-T.M.; Entenza, J.M.; Pulgarin, C. Solar photo-Fenton disinfection of 11 antibiotic-resistant bacteria (ARB) and elimination of representative AR genes. Evidence that antibiotic resistance does not imply resistance to oxidative treatment. Water Res. 2018, 143, 334–345. [Google Scholar] [CrossRef]
- Kokkinos, P.; Venieri, D.; Mantzavinos, D. Advanced oxidation processes for water and wastewater viral disinfection. a systematic review. Food Environ. Virol. 2021, 13, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Alrousan, D.M.A.; Polo-López, M.I.; Dunlop, P.S.M.; Fernández-Ibáñez, P.; Byrne, J.A. Solar photocatalytic disinfection of water with immobilised titanium dioxide in re-circulating flow CPC reactors. Appl. Catal. B Environ. 2012, 128, 126–134. [Google Scholar] [CrossRef]
- Hosseini, F.; Assadi, A.A.; Nguzen-Tri, P.; Ali, I.; Rtimi, S. Titanium-based photocatalytic coatings for bacterial disinfection: The shift from suspended powders to catalytic interfaces. Surf. Interfaces 2022, 32, 102078. [Google Scholar] [CrossRef]
- Mehrabadi, A.R.; Kardani, N.; Fazeli, M.; Hamidian, L.; Mousavi, A.; Salmani, N. Investigation of water disinfection efficiency using titanium dioxide (TiO2) in permeable to sunlight tubes. Desalination Water Treat. 2011, 28, 17–22. [Google Scholar] [CrossRef]
- Izumi, I.; Fan, F.-R.F.; Bard, A.J. Heterogeneous photocatalytic decomposition of benzoic acid and adipic acid on platinized titanium dioxide powder. The photo-Kolbe decarboxylative route to the breakdown of the benzene ring and to the production of butane. J. Phys. Chem. 1981, 85, 218–223. [Google Scholar] [CrossRef]
- Pelizzetti, E.; Minero, C. Mechanism of the photo-oxidative degradation of organic pollutants over TiO2 particles. Electrochim. Acta 1993, 38, 47–55. [Google Scholar] [CrossRef]
- Rincón, A.G.; Pulgarin, C. Photocatalytical inactivation of E. coli: Effect of (continuous–intermittent) light intensity and of (suspended–fixed) TiO2 concentration. Appl. Catal. B Environ. 2003, 44, 263–284. [Google Scholar] [CrossRef]
- Jiang, X.; Manawan, M.; Feng, T.; Qian, R.; Zhao, T.; Zhou, G.; Kong, F.; Wang, Q.; Dai, S.; Pan, J.H. Anatase and rutile in evonik aeroxide P25: Heterojunctioned or individual nanoparticles? Catal. Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Huang, Z.; Maness, P.-C.; Blake, D.M.; Wolfrum, E.J.; Smolinski, S.L.; Jacoby, W.A. Bactericidal mode of titanium dioxide photocatalysis. J. Photochem. Photobiol. A Chem. 2000, 130, 163–170. [Google Scholar] [CrossRef]
- Danwittayakul, S.; Songngam, S.; Sukkasi, S. Enhanced solar water disinfection using ZnO supported photocatalysts. Environ. Technol. 2020, 41, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Vilhunen, S.; Sillanpää, M. Recent developments in photochemical and chemical AOPs in water treatment: A mini-review. Rev. Environ. Sci. Biotechnol. 2010, 9, 323–330. [Google Scholar] [CrossRef]
- Crawford, M.H.; Banas, M.A.; Ross, M.P.; Ruby, D.S.; Nelson, J.S.; Boucher, R.; Allerman, A.A. Final LDRD report: Ultraviolet water purification systems for rural environments and mobile applications. Sandia Rep. 2005, 1, 35. [Google Scholar]
- Muramoto, Y.; Kimura, M.; Nouda, S. Development and future of ultraviolet light-emitting diodes: UV-LED will replace the UV lamp. Semicond. Sci. Technol. 2014, 29, 084004. [Google Scholar] [CrossRef]
- Würtele, M.A.; Kolbe, T.; Lipsz, M.; Külberg, A.; Weyers, M.; Kneissl, M.; Jekel, M. Application of GaN-based ultraviolet-C light emitting diodes—UV LEDs—For water disinfection. Water Res. 2011, 45, 1481–1489. [Google Scholar] [CrossRef]
- Chatterley, C.; Linden, K.G. UV-LED irradiation technology for point-of-use water disinfection. Proc. Water Environ. Fed. 2009, 2009, 222–225. [Google Scholar] [CrossRef]
- Vilhunen, S.; Särkkä, H.; Sillanpää, M. Ultraviolet light-emitting diodes in water disinfection. Environ. Sci. Pollut. Res. 2009, 16, 439–442. [Google Scholar] [CrossRef]
- FEW Chemicals GmbH. FEW Chemicals GmbH: Overview. Available online: https://www.few.de/anorganische-materialien/makrospecr/uebersicht/ (accessed on 6 August 2024).
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef]
- Marugán, J.; van Grieken, R.; Pablos, C.; Sordo, C. Analogies and differences between photocatalytic oxidation of chemicals and photocatalytic inactivation of microorganisms. Water Res. 2010, 44, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Hu, J. Inactivation/reactivation of antibiotic-resistant bacteria by a novel UVA/LED/TiO2 system. Water Res. 2013, 47, 4547–4555. [Google Scholar] [CrossRef]
- Rincón, A.-G.; Pulgarin, C. Field solar E. coli inactivation in the absence and presence of TiO2: Is UV solar dose an appropriate parameter for standardization of water solar disinfection? Sol. Energy 2004, 77, 635–648. [Google Scholar] [CrossRef]
- Rincón, A.-G.; Pulgarin, C. Effect of pH, inorganic ions, organic matter and H2O2 on E. coli K12 photocatalytic inactivation by TiO2: Implications in solar water disinfection. Appl. Catal. B Environ. 2004, 51, 283–302. [Google Scholar] [CrossRef]
- Rincón, A.-G.; Pulgarin, C. Fe3+ and TiO2 solar-light-assisted inactivation of E. coli at field scale: Implications in solar disinfection at low temperature of large quantities of water. Catal. Today 2007, 122, 128–136. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. Can the photocatalyst TiO2 be incorporated into a wastewater treatment method? Background and prospects. Catal. Today 2020, 340, 334–346. [Google Scholar] [CrossRef]
- Chatterley, C.; Linden, K. Demonstration and evaluation of germicidal UV-LEDs for point-of-use water disinfection. J. Water Health 2010, 8, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Oguma, K.; Kita, R.; Sakai, H.; Murakami, M.; Takizawa, S. Application of UV light emitting diodes to batch and flow-through water disinfection systems. Desalination 2013, 328, 24–30. [Google Scholar] [CrossRef]
- Lawrie, K.; Mills, A.; Figueredo-Fernández, M.; Gutiérrez-Alfaro, S.; Manzano, M.; Saladin, M. UV dosimetry for solar water disinfection (SODIS) carried out in different plastic bottles and bags. Sens. Actuators B Chem. 2015, 208, 608–615. [Google Scholar] [CrossRef]
- Sciacca, F.; Rengifo-Herrera, J.A.; Wéthé, J.; Pulgarin, C. Dramatic enhancement of solar disinfection (SODIS) of wild Salmonella sp. in PET bottles by H2O2 addition on natural water of Burkina Faso containing dissolved iron. Chemosphere 2010, 78, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Sundin, G.W. Construction and Analysis of Photolyase Mutants of Pseudomonas aeruginosa and Pseudomonas syringae: Contribution of Photoreactivation, Nucleotide Excision Repair, and Mutagenic DNA Repair to Cell Survival and Mutability following Exposure to UV-B Radiation. Appl. Environ. Microbiol. 2001, 67, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Busse, M.M.; Becker, M.; Applegate, B.M.; Camp, J.W.; Blatchley, E.R. Responses of Salmonella typhimurium LT2, Vibrio harveyi, and Cryptosporidium parvum to UVB and UVA radiation. Chem. Eng. J. 2019, 371, 647–656. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Benabbou, A.K.; Derriche, Z.; Felix, C.; Lejeune, P.; Guillard, C. Photocatalytic inactivation of Escherischia coli. Appl. Catal. B Environ. 2007, 76, 257–263. [Google Scholar] [CrossRef]
- Cho, M.; Kim, J.-H.; Yoon, J. Investigating synergism during sequential inactivation of Bacillus subtilis spores with several disinfectants. Water Res. 2006, 40, 2911–2920. [Google Scholar] [CrossRef]
- Shang, C.; Cheung, L.M.; Ho, C.-M.; Zeng, M. Repression of photoreactivation and dark repair of coliform bacteria by TiO2-modified UV-C disinfection. Appl. Catal. B Environ. 2009, 89, 536–542. [Google Scholar] [CrossRef]
- Bowker, C.; Sain, A.; Shatalov, M.; Ducoste, J. Microbial UV fluence-response assessment using a novel UV-LED collimated beam system. Water Res. 2011, 45, 2011–2019. [Google Scholar] [CrossRef]
- Wengraitis, S.; McCubbin, P.; Wade, M.M.; Biggs, T.D.; Hall, S.; Williams, L.I.; Zulich, A.W. Pulsed UV-C disinfection of Escherichia coli with light-emitting diodes, emitted at various repetition rates and duty cycles. Photochem. Photobiol. 2013, 89, 127–131. [Google Scholar] [CrossRef]
- Hamamoto, A.; Mori, M.; Takahashi, A.; Nakano, M.; Wakikawa, N.; Akutagawa, M.; Ikehara, T.; Nakaya, Y.; Kinouchi, Y. New water disinfection system using UVA light-emitting diodes. J. Appl. Microbiol. 2007, 103, 2291–2298. [Google Scholar] [CrossRef]
- Mori, M.; Hamamoto, A.; Takahashi, A.; Nakano, M.; Wakikawa, N.; Tachibana, S.; Ikehara, T.; Nakaya, Y.; Akutagawa, M.; Kinouchi, Y. Development of a new water sterilization device with a 365 nm UV-LED. Med. Bio Eng. Comput. 2007, 45, 1237–1241. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Kavitha, B.; Reddy, P.A.K.; Kim, K.-H. TiO2-based photocatalytic disinfection of microbes in aqueous media: A review. Environ. Res. 2017, 154, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Wang, Y.; Ma, D.; Yao, G.; Yue, Q.; Gao, B.; Xu, X. A new UV source activates ozone for water treatment: Wavelength-dependent ultraviolet light-emitting diode (UV-LED). Sep. Purif. Technol. 2022, 280, 119934. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; Jery, A.E.; Khezami, L.; Assadi, A.A. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).