Measuring Biogenic Volatile Organic Compounds from Leaves Exposed to Submicron Black Carbon Using Portable Sensor
Abstract
1. Introduction
2. Methods
2.1. Black Carbon Particles
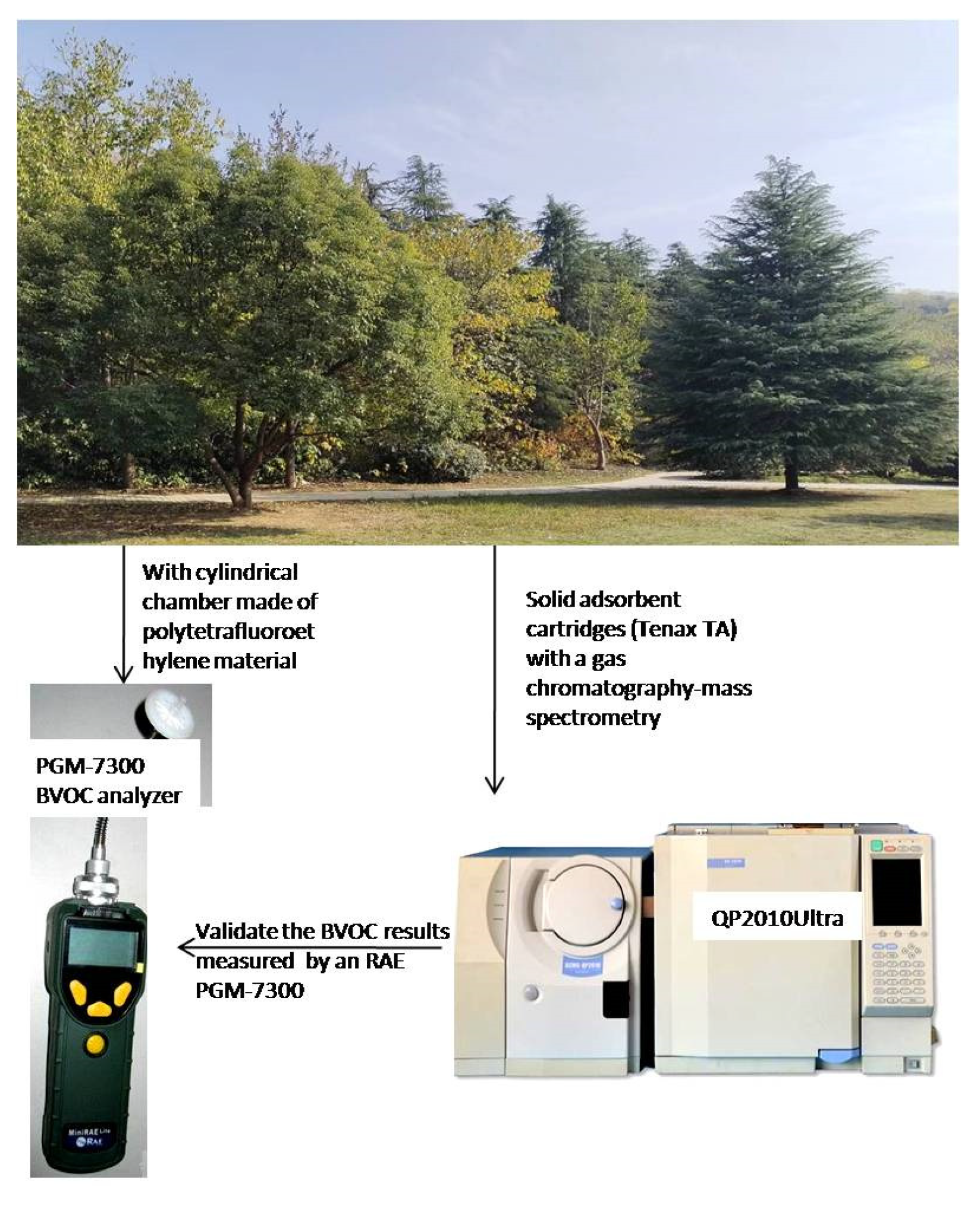
2.2. Measurements of BVOC Emission
2.3. Exposure Experiments
2.4. Statistical Analysis
3. Results and Discussion
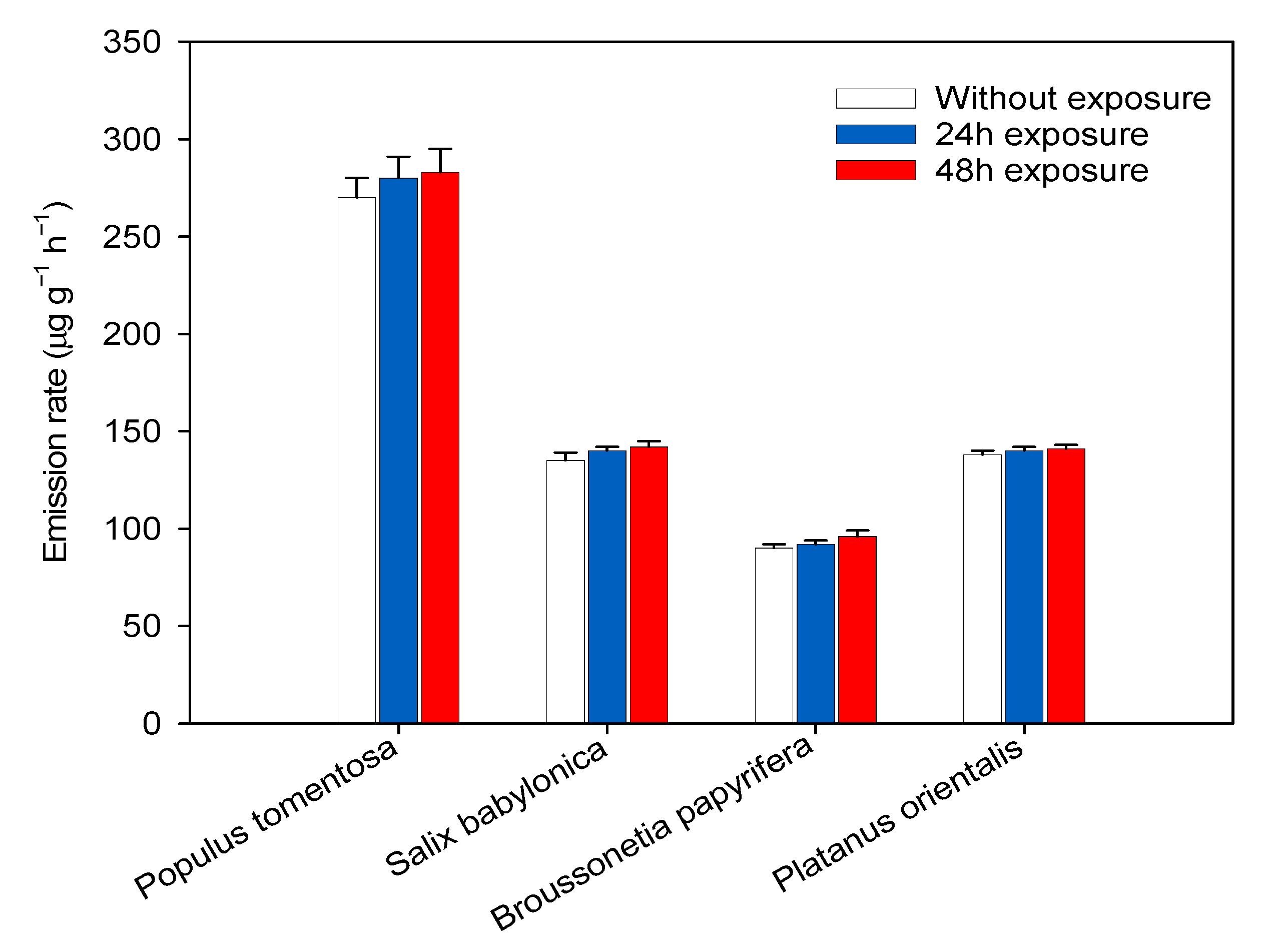
3.1. BVOC Emission across Species
3.2. The Impacts of Submicron Black Carbon
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.; Obrist, D.; Dastoor, A.; Jiskra, M.; Ryjkov, A. Vegetation uptake of mercury and impacts on global cycling. Nat. Rev. Earth Environ. 2021, 2, 269–284. [Google Scholar] [CrossRef]
- Chen, G.; Lin, L.; Hu, Y.; Zhang, Y.; Ma, K. Net particulate matter removal ability and efficiency of ten plant species in Beijing. Urban For. Urban Green. 2021, 63, 127230. [Google Scholar] [CrossRef]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C. Air pollution removal by urban trees and shrubs in the United States. Urban For. Urban Green. 2006, 4, 115–123. [Google Scholar] [CrossRef]
- Cai, M.; Xin, Z.; Yu, X. Spatio-temporal variations in PM leaf deposition: A meta-analysis. Environ. Pollut. 2017, 231, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Mori, T.; Iwamoto, Y.; Ushikubo, Y.; Miura, K. Wetting properties of fresh urban soot particles:Evaluation based on critical supersaturation and observation of surfacetrace materials. Sci. Total Environ. 2022, 811, 152274. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Baumgartner, J.; Schauer, J.J. Source apportionment of fine-particle, water-soluble organic nitrogen and its association with the inflammatory potential of lung epithelial cells. Environ. Sci. Technol. 2019, 53, 9845–9854. [Google Scholar] [CrossRef]
- Tao, M.; Xu, Y.; Liu, Q.; Liu, Y.; Tian, S.; Schauer, J.J. Penetration of submicron amino-functionalized graphene quantum dots in plant stomata, implication for the depollution of atmospheric soot particles. Environ. Chem. Lett. 2022, 21, 1281–1286. [Google Scholar] [CrossRef]
- Tao, M.; Liu, Q.; Schauer, J.J. Direct measurement of the deposition of submicron soot particles on leaves of Platanus acerifolia tree. Environ. Sci. Process. Impacts 2022, 24, 2336–2344. [Google Scholar] [CrossRef] [PubMed]
- Rindy, J.E.; Ponette-González, A.G.; Barrett, T.E.; Sheesley, R.J.; Weathers, K.C. Urban trees are sinks for soot: Elemental carbon accumulation by two widespread Oak species. Environ. Sci. Technol. 2019, 53, 10092–10101. [Google Scholar] [CrossRef]
- Wang, L.; Gong, H.; Peng, N.; Zhang, J.Z. Molecular adsorption mechanism of elemental carbon particles on leaf surface. Environ. Sci. Technol. 2018, 52, 5182–5190. [Google Scholar] [CrossRef]
- Carriero, G.; Brunetti, C.; Fares, S.; Hayes, F.; Hoshika, Y.; Mills, G.; Tattini, M.; Paoletti, E. BVOC responses to realistic nitrogen fertilization and ozone exposure in silver birch. Environ. Pollut. 2016, 213, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, R.P.; Kivimäenpää, M.; Kasurinen, A.; Häikiö, E.; Holopainen, T.; Holopainen, J.K. Herbivore-induced BVOC emissions of Scots pine under warming, elevated ozone and increased nitrogen availability in an open-field exposure. Agric. For. Meteorol. 2017, 242, 21–32. [Google Scholar] [CrossRef]
- Cao, J.; Situ, S.; Hao, Y.; Xie, S.; Li, L. Enhanced summertime ozone and SOA from biogenic volatile organic compound (BVOC) emissions due to vegetation biomass variability during 1981–2018 in China. Atmos. Chem. Phys. 2022, 22, 2351–2364. [Google Scholar] [CrossRef]
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Y.; Hu, J.; Zhang, H.; Ying, Q. Source apportionment of summer time ozone in China using a source-oriented chemical transport model. Atmos. Environ. 2019, 211, 79–90. [Google Scholar] [CrossRef]
- Li, L.Y.; Xie, S.D. Historical variations of biogenic volatile organic compound emission inventories in China, 1981–2003. Atmos. Environ. 2014, 95, 185–196. [Google Scholar] [CrossRef]
- Li, L.; Yang, W.; Xie, S.; Wu, Y. Estimations and uncertainty of biogenic volatile organic compound emission inventory in China for 2008–2018. Sci. Total Environ. 2020, 733, 139301. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, A.W.; Edwards, P.M.; Hamilton, J.F. Daytime isoprenenit rates under changing NOx and O3. Atmos. Chem. Phys. 2023, 23, 8473–8485. [Google Scholar] [CrossRef]
- Carlsson, P.T.M.; Vereecken, L.; Novelli, A.; Bernard, F.; Brown, S.S.; Brownwood, B.; Cho, C.; Crowley, J.N.; Dewald, P.; Edwards, P.M.; et al. Comparison of isoprene chemical mechanisms under atmospheric night-time conditions in chamber experiments: Evidence of hydroperoxyaldehydes and epoxyproducts from NO3 oxidation. Atmos. Chem. Phys. 2023, 23, 3147–3180. [Google Scholar] [CrossRef]
- Guion, A.; Turquety, S.; Cholakian, A.; Polcher, J.; Ehret, A.; Lathière, J. Biogenic isoprene emissions, dry deposition velocity, and surface ozone concentration during summer droughts, heat waves, and normal conditions in southwestern Europe. Atmos. Chem. Phys. 2023, 23, 1043–1071. [Google Scholar] [CrossRef]
- Mayhew, A.W.; Lee, B.H.; Thornton, J.A.; Bannan, T.J.; Brean, J.; Hopkins, J.R.; Lee, J.D.; Nelson, B.S.; Percival, C.; Rickard, A.R.; et al. Evaluation of isoprenenitrate chemistry in detailed chemical mechanisms. Atmos. Chem. Phys. 2022, 22, 14783–14798. [Google Scholar] [CrossRef]
- Bryant, D.J.; Nelson, B.S.; Swift, S.J.; Budisulistiorini, S.H.; Drysdale, W.S.; Vaughan, A.R.; Newland, M.J.; Hopkins, J.R.; Cash, J.M.; Langford, B.; et al. Biogenic and anthropogenic sources of isoprene and monoterpenes and their secondary organic aerosol in Delhi, India. Atmos. Chem. Phys. 2023, 23, 61–83. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, N.; Li, W.; Guenther, A.; Lam, J.C.Y.; Tai, A.P.K.; Potosnak, M.J.; Seco, R. Satellite-derived constraints on the effect of drought stress on biogenic isoprene emissions in the southeastern US. Atmos. Chem. Phys. 2022, 22, 14189–14208. [Google Scholar] [CrossRef]
- Dam, M.; Draper, D.C.; Marsavin, A.; Fry, J.L.; Smith, J.N. Observations of gas-phase products from the nitrate-radical-initiated oxidation of four monoterpenes. Atmos. Chem. Phys. 2022, 22, 9017–9031. [Google Scholar] [CrossRef]
- Hellén, H.; Schallhart, S.; Praplan, A.P.; Tykkä, T.; Aurela, M.; Lohila, A.; Hakola, H. Sesquiterpenes dominate monoterpenes in northern wetland emissions. Atmos. Chem. Phys. 2020, 20, 7021–7034. [Google Scholar] [CrossRef]
- Hellén, H.; Praplan, A.P.; Tykkä, T.; Helin, A.; Schallhart, S.; Schiestl-Aalto, P.P.; Bäck, J.; Hakola, H. Sesquiterpenes and oxygenated sesquiterpenes dominate the VOC(C5–C20) emissions of downy birches. Atmos. Chem. Phys. 2021, 21, 8045–8066. [Google Scholar] [CrossRef]
- Hellén, H.; Praplan, A.P.; Tykkä, T.; Ylivinkka, I.; Vakkari, V.; Bäck, J.; Petäjä, T.; Kulmala, M.; Hakola, H. Long-term measurements of volatile organic compounds highlight the importance of sesquiterpenes for the atmospheric chemistry of aboreal forest. Atmos. Chem. Phys. 2018, 18, 13839–13863. [Google Scholar] [CrossRef]
- Hoshika, Y.; Pecori, F.; Conese, I.; Bardelli, T.; Marchi, E.; Manning, W.J.; Badea, O.; Paoletti, E. Effects of a three-year exposure to ambient ozone on biomass allocation in poplar using ethylenediurea. Environ. Pollut. 2013, 180, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Hoshika, Y.; Carriero, G.; Feng, Z.; Zhang, Y.; Paoletti, E. Determinants of stomatal sluggishness in ozone-exposed deciduous tree species. Sci. Total Environ. 2014, 481, 453–458. [Google Scholar] [CrossRef]
- Yu, H.; Blande, J.D. A potential ozone defense in intercellular air space: Clues from intercellular BVOC concentrations and stomatal conductance. Sci. Total Environ. 2022, 852, 158456. [Google Scholar] [CrossRef]
- Heiden, A.C.; Hoffmann, T.; Kahl, J.; Kley, D.; Klockow, D.; Langebartels, C.; Mehlhorn, H.; Sandermann, H., Jr.; Schraudner, M.; Schuh, G.; et al. Emission of volatile organic compounds from ozone-exposed plants. Ecol. Appl. 1999, 9, 1160–1167. [Google Scholar] [CrossRef]
- Kivimäenpää, M.; Ghimire, R.P.; Sutinen, S.; Häikiö, E.; Kasurinen, A.; Holopainen, T.; Holopainen, J.K. Increases in volatile organic compound emissions of Scot spine in response to elevated ozone and warming are modified by herbivory and soil nitrogen availability. Eur. J. For. Res. 2016, 135, 343–360. [Google Scholar] [CrossRef]
- Zhu, J.; Shang, J.; Chen, Y.; Kuang, Y.; Zhu, T. Reactive oxygen species-related inside-to-outside oxidation of soot particles triggered by visible-light irradiation:Physicochemical property changes and oxidative potential enhancement. Environ. Sci. Technol. 2020, 54, 8558–8567. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sheng, M.; Shang, J.; Kuang, Y.; Shi, X.; Qiu, X. Photocatalytic role of atmospheric soot particles under visible-light irradiation: Reactive oxygen species generation, self-oxidation process, and induced higher oxidative potential and cytotoxicity. Environ. Sci. Technol. 2022, 56, 7668–7678. [Google Scholar] [CrossRef]
- Custódio, D.; Cerqueira, M.; Fialho, P.; Nunes, T.; Pio, C.; Henriques, D. Wet deposition of particulate carbon to the Central North Atlantic Ocean. Sci. Total Environ. 2014, 496, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Fu, B.; Tao, S.; Zhu, D.; Wang, X.; Peng, S.; Li, B. Impact of the initial hydrophilic ratio on black carbon aerosols in the Arctic. Sci. Total Environ. 2022, 817, 153044. [Google Scholar] [CrossRef] [PubMed]
- Karlik, J.F.; McKay, A.H.; Welch, J.M.; Winer, A.M. A survey of California plant species with a portable VOC analyzer for biogenic emission inventory development. Atmos. Environ. 2002, 36, 5221–5233. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Wang, X. Determination of dimethylsulfide and dimethylselenide in human urine by portable gas chromatography–photoionization detection with headspace sampling. Microchem. J. 2011, 99, 352–355. [Google Scholar] [CrossRef]
- Aydin, Y.M.; Yaman, B.; Koca, H.; Dasdemir, O.; Kara, M.; Altiok, H.; Dumanoglu, Y.; Bayram, A.; Tolunay, D.; Odabasi, M.; et al. Biogenic volatile organic compound(BVOC) emissions from forested areas in Turkey: Determination of specific emission rates for thirty-one tree species. Sci. Total Environ. 2014, 490, 239–253. [Google Scholar] [CrossRef]
- Calfapietra, C.; Fares, S.; Manes, F.; Morani, A.; Sgrigna, G.; Loreto, F. Role of biogenic volatile organic compounds (BVOC) emitted by urban trees on ozone concentration in cities: Areview. Environ. Pollut. 2013, 183, 71–80. [Google Scholar] [CrossRef]
- Ren, Y.; Ge, Y.; Ma, D.; Song, X.; Shi, Y.; Pan, K.; Qu, Z.; Guo, P.; Han, W.; Chang, J. Enhancing plant diversity and mitigating BVOC emissions of urban green spaces through the introduction of ornamental tree species. Urban For. Urban Green. 2017, 27, 305–313. [Google Scholar] [CrossRef]
- Cagliero, C.; Mastellone, G.; Marengo, A.; Bicchi, C.; Sgorbini, B.; Rubiolo, P. Analytical strategies for in-vivo evaluation of plant volatile emissions—A review. Anal. Chim. Acta 2021, 1147, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Lun, X.; Lin, Y.; Chai, F.; Fan, C.; Li, H.; Liu, J. Reviews of emission of biogenic volatile organic compounds (BVOCs) in Asia. J. Environ. Sci. 2020, 95, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-W.; Dinh, T.-V.; Park, S.-Y.; Choi, I.-Y.; Park, C.-R.; Son, Y.-S. Characteristics of biogenic volatile organic compounds emitted from major species of street trees and urban forests. Atmos. Pollut. Res. 2022, 13, 101470. [Google Scholar] [CrossRef]
| Methods | Linear Range (μg m−3) | Correlation Coefficients (R2) | Limits of Detection | Relative Standard Deviations | Sample 1 (n = 3, μg m−3) | Sample 2 (n = 3, μg m−3) |
|---|---|---|---|---|---|---|
| GC-MS | 0.2–50 | 0.99 | 0.05 | 3.2 | 34 ± 2 | 15 ± 1 |
| PGM-7300 analyzer | 10–500 | 0.98 | 5 | 4.8 | 39 ± 5 | 20 ± 4 |
| Species | BVOC Emissions (μg g−1 h−1) | 2 μg mL−1 | p-Value | 5 μg mL−1 | p-Value | 10 μg mL−1 | p-Value |
|---|---|---|---|---|---|---|---|
| Populus tomentosa | 270 ± 10 | 285 ± 13 | 0.08 | 280 ± 15 | 0.09 | 287 ± 9 | 0.07 |
| Salix babylonica | 135 ± 4 | 143 ± 2 | 0.10 | 145 ± 2 | 0.11 | 138 ± 4 | 0.12 |
| Broussonetia papyrifera | 90 ± 2 | 92 ± 3 | 0.07 | 94 ± 2 | 0.08 | 95 ± 2 | 0.09 |
| Platanus orientalis | 138 ± 2 | 140 ± 2 | 0.18 | 142 ± 2 | 0.11 | 144 ± 3 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Liu, Y. Measuring Biogenic Volatile Organic Compounds from Leaves Exposed to Submicron Black Carbon Using Portable Sensor. Pollutants 2024, 4, 187-195. https://doi.org/10.3390/pollutants4020012
Liu Q, Liu Y. Measuring Biogenic Volatile Organic Compounds from Leaves Exposed to Submicron Black Carbon Using Portable Sensor. Pollutants. 2024; 4(2):187-195. https://doi.org/10.3390/pollutants4020012
Chicago/Turabian StyleLiu, Qingyang, and Yanju Liu. 2024. "Measuring Biogenic Volatile Organic Compounds from Leaves Exposed to Submicron Black Carbon Using Portable Sensor" Pollutants 4, no. 2: 187-195. https://doi.org/10.3390/pollutants4020012
APA StyleLiu, Q., & Liu, Y. (2024). Measuring Biogenic Volatile Organic Compounds from Leaves Exposed to Submicron Black Carbon Using Portable Sensor. Pollutants, 4(2), 187-195. https://doi.org/10.3390/pollutants4020012







