Abstract
Calcium aluminate slag produced by the aluminothermic reduction of silica is tested as a candidate raw material for the hydrometallurgical production of pure aluminium chloride hexahydrate (ACH) through leaching with hydrochloric acid. The crystallization of ACH follows by sparging the pregnant liquor with hydrochloric gas. Almost total extraction of Al is achieved with the use of azeotropic HCl acid solution (5.9 M) at 80 °C and 1 h retention time. A pregnant liquor with approximately 20 wt% AlCl3 is produced as a base for ACH crystallization by sparging it with gaseous HCl. The ACH produced is re-dissolved and crystallized three to four times until high purity is achieved. High purity ACH acts as a precursor for producing High Purity Alumina (HPA), a high added value material used in LEDs and lithium-ion batteries and other niche applications.
1. Introduction
The SisAl Pilot EU H2020 project targets the sustainable production of silicon alloys and high purity alumina, utilizing secondary silicon dioxide and aluminium bearing resources. It demonstrates a patented novel industrial process to produce silicon alloys, enabling a shift from today’s carbothermic submerged arc furnace (SAF) process to a far more environmentally benign and hopefully economically viable alternative: an aluminothermic reduction of quartz that utilizes secondary raw materials such as aluminium (Al) scrap and dross, effectively de-carbonizing the process.
Primary and secondary Al are major material flows in our society. Aluminium is the second most used metal, after steel, and Al production is larger than all other non-ferrous metals in total, with strong growth expected up to 2050 [1]. Utilizing Al-dross for the aluminothermic reduction of quartz and producing metallic silicon and calcium aluminate slag is a resource efficient alternative to both the existing silicon alloy and dross recycling technologies available on the market today. Detailed information about the dross and its performance in the aluminothermic process has been published by Philipson et al. [2]. The SisAl process produces an alumina-rich calcium aluminate slag which can be used as resource for pure alumina extraction. Calcium aluminate slags similar to the one produced in the SisAl process can be processed with alkaline leaching to extract metallurgical grade alumina, in accordance with previous established industrial practices [3]. Alternative to this alkaline route is the hydrochloric acid (HCl) route, examined in this work. Acid leaching using hydrochloric acid (HCl), offers several advantages such as high metal solubility, enhanced redox potentials and high leaching rates, that have prompted intensive investigation of the HCl-clay process [4]. One of the main advantages of acid leaching for producing alumina is the selective crystallization process of AlCl3·6H2O by sparging HCl gas to the pregnant leaching solution (PLS). In this case, the precipitation of aluminium chloride hexahydrate (AlCl3·6H2O) is obtained by saturating the solution with hydrogen chloride gas. The AlCl3·6H2O can be further calcined to recover HCl and produce alumina.
The leaching of CaO-Al2O3-based slag with HCl solution is a complicated process with the following chemical reactions as the major reactions in the leaching process:
Acid leaching of calcium aluminate slags has not been extensively studied by the international literature, however HCl treatment of different type of materials is reported as an appropriate method for Al extraction. Aglieti et al. studied an acid process for the recovery of alumina of over 98.7% purity from aluminous red soils of Argentina. The concentration of hydrochloric acid was 6M and leaching was carried out at 100 °C with a stoichiometric molar ratio acid/aluminium of 1.5. The maximum recovery of Al after 60 min was 50% and in 180 min the recovery reached 84% [5]. Yang et al. investigated the leaching kinetics of aluminium extraction from secondary aluminium dross by using 3.29 M HCl for 120 min. Leaching test results showed that the leaching extent of aluminium reached 20% with an initial solid/liquid ratio 1:10 [6].
High purity alumina (HPA; purity > 99.99%) is a critical ingredient for clean-tech products such as LED, Li-ion Batteries, and many other high-value niche applications (e.g., abrasives, catalysts and specialty sapphire). These applications drive a strong market growth and there is a need for sustainable technologies to produce HPA in large quantities. One simple method to obtain HPA is the calcination of purified aluminium chloride hexahydrate (ACH). Hence, the crystallization of ACH, which is also a purification process, becomes important to produce high added value materials. In the past, it has been used to obtain metallurgical grade alumina from clay minerals [7], while within the SisAl project it will be further developed as a refining process.
2. Materials and Methods
2.1. Materials
Prior to leaching, calcium aluminate slag (Al2O3-CaO) was produced, along with silicon alloy, by aluminothermic reduction of calcium silicate slag at NTNU in Trondheim, Norway. Aluminum dross was used as reductant in the aluminothermic reduction of SiO2 in a CaO-SiO2 slag [2]. Dross was received from Hydro Aluminium Sunndaløra. Prior to aluminothermic reduction, the metallic Al content in the dross was estimated at 72 wt%, which subsequently was used for calculating the dross to slag weight ratio. Pre-fused CaO-SiO2 slag was dried and milled before being analysed by XRF. The resulting composition was 51.3 wt%. CaO and 46. 7 wt% SiO2.
In a graphite crucible with inner dimensions Ø11 cm × H 35 cm and a graphite foil mounted along the interior walls, 812 g of dross of particle size 5–10 mm was covered by 1993 g of pre-fused CaO-SiO2 slag and melted in an induction furnace with air atmosphere. The content was heated for 34 min up to 1650 °C and held between 1650 and 1750 °C for one hour, requiring power input between 13 and 17 kW. The crucible with content was slowly cooled inside the furnace to room temperature. The temperature reached 800 °C after approximately 40 min. The graphite foil made separation of the product from crucible possible. Si alloy and slag were then separated by a hammer. The chemical composition was analysed by SisAl partners, University of Aachen (RWTH), with RFA WROXI XRF, Elkem with WD XRF and NTUA with ED XRF to increase the validity of the composition determined. In addition, the metallic Si content in the slag was determined by Elkem through a wet chemical method. Slag with no visible bulk Si alloy entrapment was milled to powder (100%–150 μm) and sent to NTUA for alumina extraction. In addition, the slag was analysed chemically by EDXRF using an Energy Dispersive X-ray Fluorescence instrument Xepos (SPECTRO A.I. GmbH company, Kleve, Germany) and P-XRD by X-ray Diffractometry and mineralogically using a SIEMENS D5000 Diffractometer with Cu Kα1 (Ni filtered) radiation, in the 2ndeta range from 2 to 75° and 0.02°/s step XRD analysis.
2.2. Acid Leaching of Slag
Hydrochloric acid (Sigma-Aldrich 37%, 1.18 g·mL−1) was used as the leaching medium. The desired concentration of HCl was prepared by dilution with de-ionized water. The experiments were carried out using a 250 mL four-necked round bottom flask placed in a thermostatically controlled mantle. A thermocouple was fitted to one of the openings to constantly control the temperature. Calcium aluminate slag was added to 150 mL agitated HCl azeotropic solution 5.9 M in a solid/liquid ratio equal to 1:10 g·mL−1, at a 80 °C. The reaction mixture was stirred at 300 revolutions per minute throughout the experiment. At selected time intervals, 5 mL of solution was taken and filtered using a filter 0.45 μm pore size. The experiments were conducted in duplicates. The leachate was analysed to determine Al, Ca, Si and Fe content in the solution, while the filter cake was dried in an oven at 100 ± 10 °C for 24 h, followed by cooling at room temperature to remove any entrapped moisture leading to weight difference. Then, the dried sample was uniformly ground and evaluated mineralogically and chemically through X-ray diffractometer (XRD), Bruker D8-Focus with CuKa radiation with nickel filter (k = 1.5405 Å) and X-ray fluorescence (XRF) analysis using the XEPOS spectrophotometer with X-LabPro software, respectively.
2.3. Aluminum Chloride Hexahydrate Precipitation
As already mentioned, the crystallization of ACH by HCl sparging is a purification process. Hereby gaseous HCl is injected into a pregnant leaching solution containing Al ions. The increasing HCl content in the liquid decreases the solubility of aluminium chloride and, as a result, ACH purer than the starting solution is selectively precipitated. Figure 1 depicts the process flow of this refining technique. The process can be repeated by re-dissolving the ACH in pure water and recrystallizing it until the desired purity is reached.
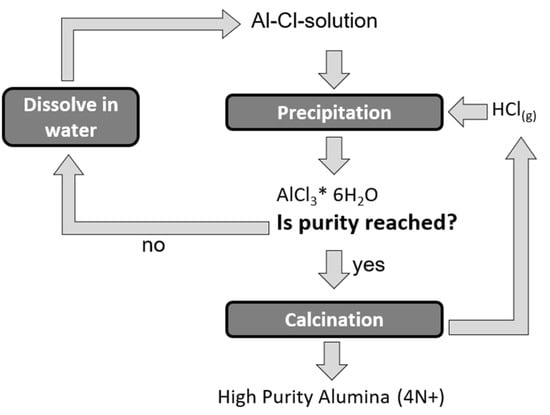
Figure 1.
Process flow of ACH purification.
3. Results and Discussion
3.1. Aluminothermic Reduction Experiments
The resulting elemental composition (wt%) in the slag after reduction is presented in Table 1, measured by WD-XRF on fused beads. Vertical cross section observation showed a well separated Si alloy in the shape of a hemisphere on top of the slag. The slag mainly consists of CaO and Al2O3, including unreacted SiO2. In addition, the slag contains significant amount of metallic Si and SiC. The quantified metallic Si content was 2.0 wt%, an indicator of the separation ability of the two products. The results show that Si alloy and slag high in alumina are rapidly produced by reacting dross with CaO-SiO2 fused slag even though the experimental scale is increased, compared to experiments conducted on a smaller scale with argon atmosphere and lower temperatures at 1650 °C [2]. The green colour of the slag in addition to XRD results indicate that fine SiC particles are homogenously distributed in the slag. Although difficult to quantify, the lower weight of the Si alloy (relative to its experimental scale) and somewhat higher Si content in the slag indicates less aluminothermic reaction compared to Philipson et al. [2].

Table 1.
Chemical analysis of the slag produced by aluminothermic reduction.
3.2. Characterization of Produced Slag
The mineralogical analysis (XRD) of the raw material and the leaching residue is presented in Figure 2.
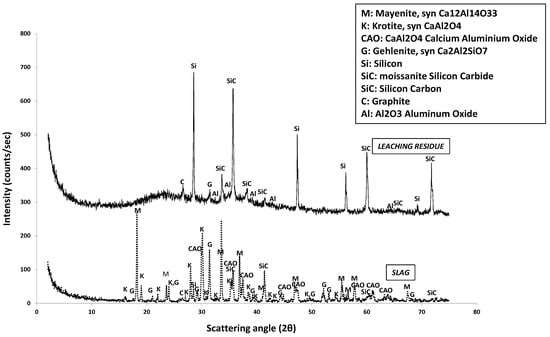
Figure 2.
XRD pattern of raw Ca-Al slag material and XRD pattern of the residue after the acid leaching.
Figure 2 shows that krotite (CaAl2O4), mayenite (Ca12Al14O33) and gehlenite (Ca2Al2SiO7) are the major mineral phases. Silicon carbide (SiC) and graphite (C) are also detected. According to Figure 2, the main mineralogical phases of the leaching residue are metallic silicon (Si), silicon carbide (SiC) and graphite (C). Thus, the residue contains some remaining impurities from the raw material, while the main phases are not visible, indicating complete Ca and Al dissolution.
3.3. Evaluation of PLS
Figure 3 shows the Al, Ca, Fe and Si recovery from the acid leaching process. The results show a 100% recovery of Al (26,785 mg/L) and Ca (29,510 mg/L) from the very first hour and, at the same time, a low recovery of silicon (60 mg/L) and iron (13 mg/L). The low-level contaminated solution, intended for crystallization process. However, calcium will be the main concern in the purification stage, urging the need of further treatment to be suitable for the targeted purposes.
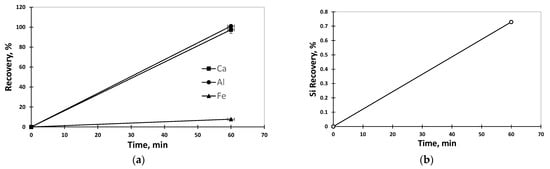
Figure 3.
Time evolution of (a) Al recovery Ca and Fe recovery and (b) time evolution of Si recovery after the leaching with 5.87 M HCl, at 80 °C, agitation 300 rpm for 1 h, solid to liquid ratio (S/L) 1/10.
3.4. ACH Precipitation
How precipitation of ACH can reveal a high purity to obtain HPA after several cycles of re-crystallization is the subject of ongoing studies. Hereby, the yield of a single step has to be kept high and the total number of steps should be low. At the same time, the purification effect must be maximized, which becomes an optimization task with numerous aspects.
To study the yield of a single step, the solubility of aluminium chloride as a function of HCl concentration must be understood. Figure 4 shows the dependence of this solubility with own data in comparison to the literature.
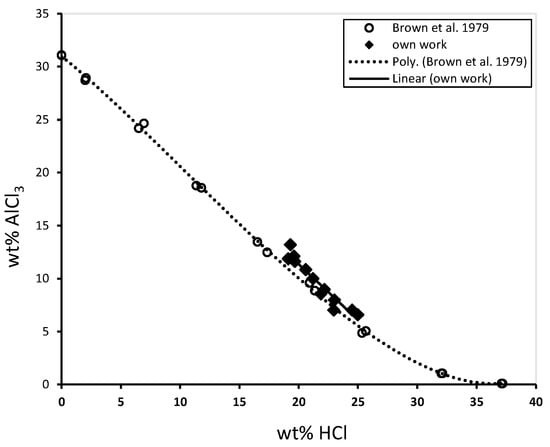
Figure 4.
Solubility of aluminium chloride as a function of HCl concentration.
It becomes apparent that at HCl concentration between 25 and 30 wt%. the residual dissolved aluminium chloride is between 2 and 5 wt%. This suggests a high yield of the step, since most of the aluminium chloride has been precipitated. However, at high HCl concentration, co-precipitation of other dissolved impurities may occur. This will be further investigated in the SisAl Project, i.e., how purification and yield can be optimized.
4. Conclusions
In this research, calcium aluminate slag produced after aluminothermic reduction was leached in HCl solutions and, subsequently, aluminium was precipitated as AlCl3∙6H2O (aluminium chloride hexahydrate or ACH) using HCl gas. The production of high purity alumina can be achieved via the acidic route by the crystallization of ACH followed by calcination. The correlation between purity and yield must be optimized, which is the subject of further studies. However, the sisal process proved to be an economically viable and environmentally sustainable alternative to the conventional carbothermic reaction, lowering CO2 emissions and promoting circularity, converting industrial by-products into high added value materials such as HPA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The research leading to these results has been performed within the SisAl Pilot project (https://www.sisal-pilot.eu/ (accessed on 1 May 2020)) and received funding from the European Community’s Horizon 2020 Programme (H2020/2014-2020) under grant agreement No 869268.
References
- Cullen, J.M.; Allwood, J.M. Mapping the global flow of aluminum: From liquid aluminum to end-use goods. Environ. Sci. Technol. 2013, 47, 3057–3064. [Google Scholar] [CrossRef] [Green Version]
- Philipson, H.; Wallin, M.; Einarsrud, K.E.; Tranell, G. Kinetics of silicon production by aluminothermic reduction of silica using aluminium and aluminium dross as reductants. In Infacon XVI: International Ferro-Alloys Congress; Waernes, G.T.A.N., Tangstad, M., Ringdalen, E., van der Eijk, C., Eds.; SINTEF/NTNU/FFF: Trondheim, Norway, 2021. [Google Scholar]
- Vafeias, M.; Dimitrios Panias, D.M.; Jafar, S.; van der Eijk, C.; Balomenos, E.; Ksiazek, M.; Davris, P. From red to grey revisiting the Pedersen process to achieve holistic bauxite ore utilization.pdf. In Proceedings of the 2nd International Bauxite Residue Valorisation and Best Practices Conference, Athens, Greece, 7–10 May 2018. [Google Scholar]
- Sanda, O.; Tinubu, A.C.; Taiwo, E.A. Recovery of iron from EAF smelter slags via hydrochloric acid leaching and solvent extraction using trioctyl phosphine oxide. Sep. Sci. Technol. 2020, 56, 1026–1034. [Google Scholar] [CrossRef]
- Aglietti, E.F.; Tavani, E.L.; Lopez, J.P. Acid process for the extraction of aluminium from argentine weathered basalts. Miner. Eng. 1990, 3, 331–343. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Q.; Zhang, G.; Shi, Q.; Feng, H. Investigation of leaching kinetics of aluminum extraction from secondary aluminum dross with use of hydrochloric acid. Hydrometallurgy 2019, 187, 158–167. [Google Scholar] [CrossRef]
- James, I.; Hoffman, R.T.L.; Harold, J.; Lwis Jesse Clark, C.; John Drake, H. Development of a hydrochloric acid process for the production of Alumina from clay. Natl. Bereau Stand. 1946, 37, 409–428. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).