Sustainable Supply of Scandium for the EU Industries from Liquid Iron Chloride Based TiO2 Plants †
Abstract
:1. Introduction
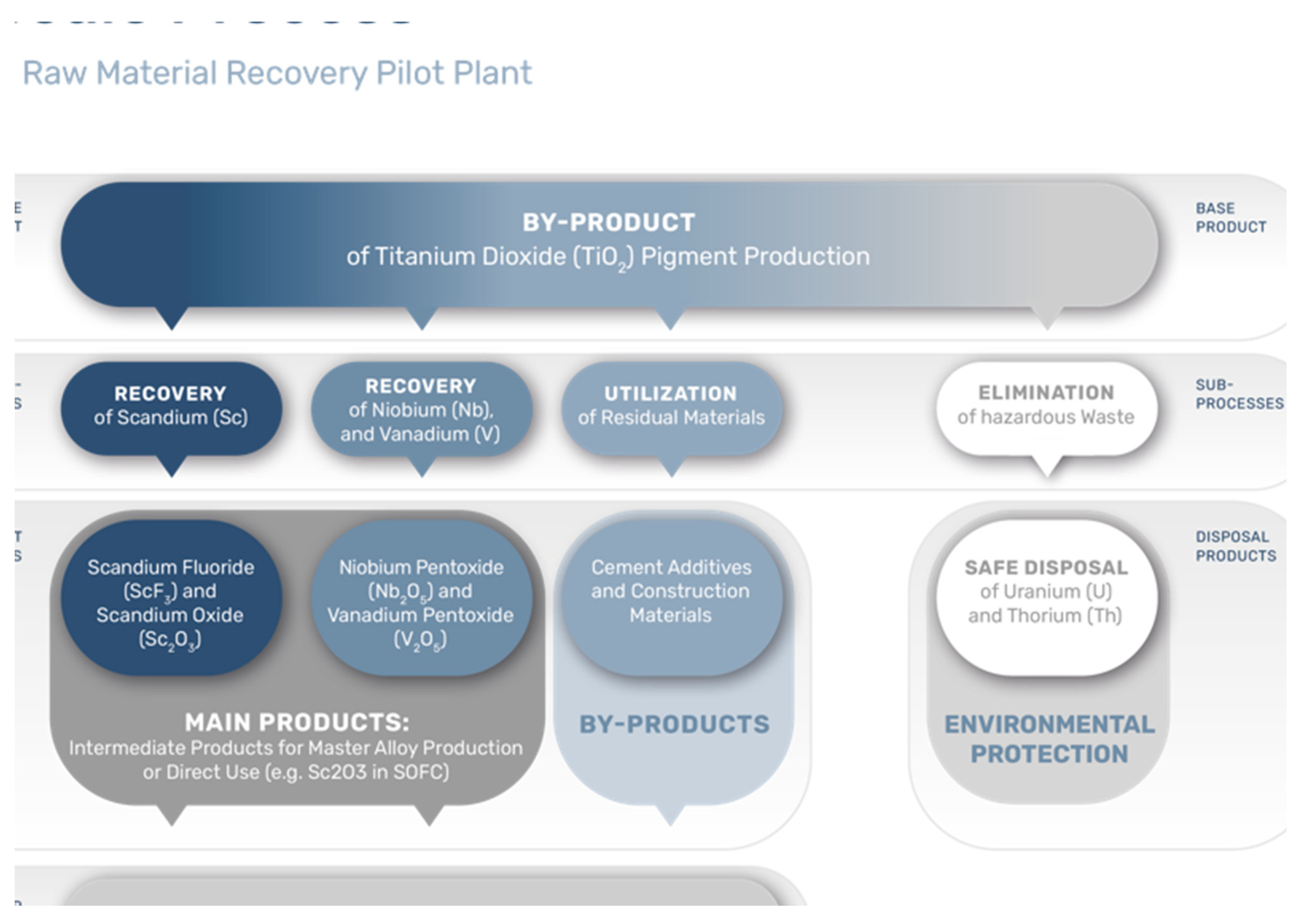
2. Scandium Resources from TiO2 Pigment Production
3. Scandium Markets
3.1. The Exceptional Properties of Scandium
3.2. The Major Scandium Markets and Applications
3.3. Scandium Suppliers at Present and in Future
3.4. Scandium Products and Price Evolution
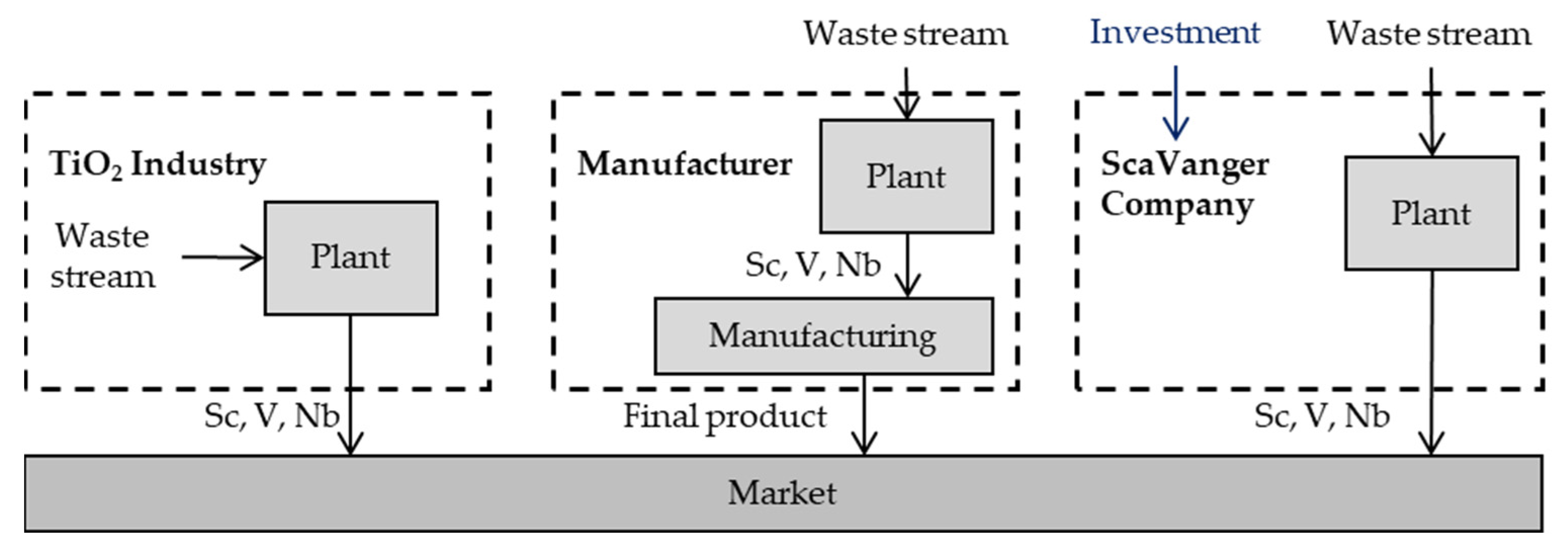
4. The ScaVanger Technology
4.1. Metal Extraction from Lab to Pilot from TRL 2 to TRL 5-6
4.2. From Pilot to Industrial Scale TRL5-6 to TRL 8-9 (Go-to-Market)
4.3. ScaVanger as Business Partner
5. Outlook
- A massive uptake potential of Sc in electricity producing technologies (SOFC, SOEC), heat exchanger and aircraft sectors necessitates continuous, high-quality Sc production in form of intermediate and refined Sc products in Europe at competitive prices compared to China.
- There is a significant potential in the EU for Sc production from TiO2 pigment production. The complete Sc need for various markets could be supplied from this promising source.
- The Sc resource from TiO2 pigment production residues depends on the evolution of the TiO2 market. It can be expected that no new TiO2 producer arrives on the European market despite forecasts for a global increase in TiO2 oxide production. TiO2 production from the chloride method can be considered constant and remains a reliable and continuous sustainable Sc source.
- The innovative ScaVanger processing method proposes a complete, feasible, greener and adaptable process for simultaneous Sc, Nb and V compound manufacturing since the supply of these elements is critical for the future. ScaVanger can be classified as a zero-waste generating process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dorin, T.; Ramajayam, M.; Vahid, A.; Langan, T. Aluminium scandium alloys. In Fundamentals of Aluminium Metallurgy: Recent Advances; Elsevier: Duxford, UK, 2018; pp. 439–494. [Google Scholar]
- Kiemel, S.; Smolinka, T.; Lehner, F.; Full, J.; Sauer, A.; Miehe, R. Critical materials for water electrolysers at the example of the energy transition in Germany. Int. J. Energy Res. 2021, 45, 9914–9935. [Google Scholar] [CrossRef]
- Yamamoto, O.; Arati, Y.; Takeda, Y.; Imanishi, N.; Mizutani, Y.; Kawai, M.; Nakamura, Y. Electrical conductivity of stabilized zirconia with ytterbia and scandia. Solid State Ion. 1995, 79, 137–142. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.; Skinner, S.; Kilner, J. Performance of solid oxide electrolysis cells based on scandia stabilised zirconia. J. Power Sources 2009, 192, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy 2011, 108, 100–108. [Google Scholar] [CrossRef]
- Titanium, Dioxide, Manufacturers & Association. The Economic Impact of Titanium Dioxide in Europe. Available online: https://tdma.info/the-economic-impact-of-titanium-dioxide-in-europe/ (accessed on 12 July 2021).
- Gambogi, J. USGS Minerals Information: Titanium and Titanium Dioxide; (Online); U.S. Geological Survey, National Minerals Information Center: Reston, VA, USA, 2021.
- Zhou, J.; Ning, S.; Meng, J.; Zhang, S.; Zhang, W.; Wang, S.; Chen, Y.; Wang, X.; Wei, Y. Purification of scandium from concentrate generated from titanium pigments production waste. J. Rare Earths 2021, 39, 194–200. [Google Scholar] [CrossRef]
- Zou, D.; Li, H.; Chen, J.; Li, D. Recovery of scandium from spent sulfuric acid solution in titanium dioxide production using synergistic solvent extraction with D2EHPA and primary amine N1923. Hydrometallurgy 2020, 197, 105463. [Google Scholar] [CrossRef]
- Letendre, S. Rio Tinto Réalise une Première Vente D’alliage Aluminium-Scandium Pour L’impression 3D. Available online: https://www.riotinto.com/can/news/releases/2021/Rio-Tinto-ralise-une-premire-vente-dalliage-aluminium-scandium-pour-limpression-3D (accessed on 10 July 2021).
- Qiu, H.; Wang, M.; Xie, Y.; Song, J.; Huang, T.; Li, X.-M.; He, T. From trace to pure: Recovery of scandium from the waste acid of titanium pigment production by solvent extraction. Process Saf. Environ. Prot. 2019, 121, 118–124. [Google Scholar] [CrossRef]
- Middlemas, S.; Fang, Z.Z.; Fan, P. Life cycle assessment comparison of emerging and traditional Titanium dioxide manufacturing processes. J. Clean. Prod. 2015, 89, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Marquis, E.; Seidman, D. Nanoscale structural evolution of Al3Sc precipitates in Al (Sc) alloys. Acta Mater. 2001, 49, 1909–1919. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, M.; Cross, C.; Grong, Ø. Effect of scandium and titanium–boron on grain refinement and hot cracking of aluminium alloy 7108. Sci. Technol. Weld. Join. 1999, 4, 381–388. [Google Scholar] [CrossRef]
- Røyset, J.; Ryum, N. Scandium in aluminium alloys. Int. Mater. Rev. 2005, 50, 19–44. [Google Scholar] [CrossRef]
- Nieh, T.; Hsiung, L.; Wadsworth, J.; Kaibyshev, R. High strain rate superplasticity in a continuously recrystallized Al—6% Mg—0.3% Sc alloy. Acta Mater. 1998, 46, 2789–2800. [Google Scholar] [CrossRef]
- Seidman, D.N.; Marquis, E.A.; Dunand, D.C. Precipitation strengthening at ambient and elevated temperatures of heat-treatable Al (Sc) alloys. Acta Mater. 2002, 50, 4021–4035. [Google Scholar] [CrossRef]
- Arachi, Y.; Sakai, H.; Yamamoto, O.; Takeda, Y.; Imanishai, N. Electrical conductivity of the ZrO2–Ln2O3 (Ln= lanthanides) system. Solid State Ion. 1999, 121, 133–139. [Google Scholar] [CrossRef]
- Sridhar, K.; Mcelroy, J.F.; Finn, J.E.; Mitlitsky, F.; Gottmann, M. Co-Production of Hydrogen and Electricity in a High Temperature Electrochemical System. U.S. Patent US7482078B2, 27 January 2009. [Google Scholar]
- McElroy, J.; Gottmann, M. Solid Oxide Regenerative Fuel Cell for Airplane Power Generation and Storage. U.S. Patent US6854688B2, 15 February 2005. [Google Scholar]
- Samsonov, G.V.; Makarenko, G.; Kosolapova, T.Y. Scandium carbides and compound scandium-titanium carbides. Doklady Akad. Nauk SSSR. 1962, 144, 1062–1065. [Google Scholar]
- Kaminskii, A.A. Laser Crystals: Their Physics and Properties; Springer: Berlin/Heidelberg, Germany, 2013; Volume 14. [Google Scholar]
- Hibst, R.; Keller, U. Experimental studies of the application of the Er: YAG laser on dental hard substances: I. Measurement of the ablation rate. Lasers Surg. Med. 1989, 9, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Rehman, F.; Chaturvedy, V. Soft tissue applications of Er, Cr: YSGG laser in pediatric dentistry. Int. J. Clin. Pediatric Dent. 2017, 10, 188. [Google Scholar]
- Keeffe, W. Recent progress in metal halide discharge-lamp research. IEE Proc. A-Phys. Sci. Meas. Instrum. Manag. Educ. -Rev. 1980, 127, 181–189. [Google Scholar] [CrossRef]
- Gambogi, J. USGS Minerals Information: Scandium; (Online); U.S. Geological Survey, National Minerals Information Center: Reston, VA, USA, January 2020; pp. 144–145.
- Laan, H.v.d. Robust scandium supply chain for aerospace applications. In Proceedings of the European Scandium Inventory Workshops, Berlin, Germany, 26–27 November 2018. [Google Scholar]
- Weber, A.; Ivers-Tiffée, E. Materials and concepts for solid oxide fuel cells (SOFCs) in stationary and mobile applications. J. Power Sources 2004, 127, 273–283. [Google Scholar] [CrossRef]
- Farrow, C. How To Make TiO2 Manufacturing More Efficient. Available online: http://www.gsa-env.co.uk/news/how-to-make-tio2-manufacturing-more-efficient/ (accessed on 10 July 2021).
- Anactisis. Home Page. Available online: https://anactisis.com/ (accessed on 10 July 2021).
- Peters, E.M.; Dittrich, C.; Yagmurlu, B.; Forsberg, K. Co-precipitation of Impurity (Ti, Fe, Al, Zr, U, Th) Phases during the Recovery of (NH 4) 3 ScF 6 from Strip Liquors by Anti-solvent Crystallization. In Rare Metal Technology 2020; Springer: New York, NY, USA, 2020; pp. 177–189. [Google Scholar]
- Yagmurlu, B.; Zhang, W.; Heikkila, M.J.; Koivula, R.T.; Friedrich, B. Solid-State Conversion of Scandium Phosphate into Scandium Oxide with Sodium Compounds. Ind. Eng. Chem. Res. 2019, 58, 14609–14620. [Google Scholar] [CrossRef]
- Yagmurlu, B.; Dittrich, C.; Friedrich, B. Innovative scandium refining processes from secondary raw materials. In Proceedings of the European Scandium Inventory Workshops, Berlin, Germany, 26–27 November 2018. [Google Scholar]
- Yagmurlu, B.; Dittrich, C.; Dunn, G. Innovative Scandium Recovery Method from Metallic End Life Products. In Proceedings of the ERES 2020, Online, 6–9 October 2020. [Google Scholar]
- Kamat, G.; Gupta, C. Open aluminothermic reduction of columbium (Nb) pentoxide and purification of the reduced metal. Metall. Trans. 1971, 2, 2817–2823. [Google Scholar] [CrossRef]
- Jaroni, M.S.; Friedrich, B.; Letmathe, P. Economical feasibility of rare earth mining outside China. Minerals 2019, 9, 576. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagmurlu, B.; Orberger, B.; Dittrich, C.; Croisé, G.; Scharfenberg, R.; Balomenos, E.; Panias, D.; Mikeli, E.; Maier, C.; Schneider, R.; et al. Sustainable Supply of Scandium for the EU Industries from Liquid Iron Chloride Based TiO2 Plants. Mater. Proc. 2021, 5, 86. https://doi.org/10.3390/materproc2021005086
Yagmurlu B, Orberger B, Dittrich C, Croisé G, Scharfenberg R, Balomenos E, Panias D, Mikeli E, Maier C, Schneider R, et al. Sustainable Supply of Scandium for the EU Industries from Liquid Iron Chloride Based TiO2 Plants. Materials Proceedings. 2021; 5(1):86. https://doi.org/10.3390/materproc2021005086
Chicago/Turabian StyleYagmurlu, Bengi, Beate Orberger, Carsten Dittrich, Georges Croisé, Robin Scharfenberg, Efthymios Balomenos, Dimitrios Panias, Eleni Mikeli, Carolin Maier, Richard Schneider, and et al. 2021. "Sustainable Supply of Scandium for the EU Industries from Liquid Iron Chloride Based TiO2 Plants" Materials Proceedings 5, no. 1: 86. https://doi.org/10.3390/materproc2021005086
APA StyleYagmurlu, B., Orberger, B., Dittrich, C., Croisé, G., Scharfenberg, R., Balomenos, E., Panias, D., Mikeli, E., Maier, C., Schneider, R., Friedrich, B., Dräger, P., Baumgärtner, F., Schmitz, M., Letmathe, P., Sakkas, K., Georgopoulos, C., & van den Laan, H. (2021). Sustainable Supply of Scandium for the EU Industries from Liquid Iron Chloride Based TiO2 Plants. Materials Proceedings, 5(1), 86. https://doi.org/10.3390/materproc2021005086






