Abstract
The present paper gives the thus far unpublished results of a pilot-scale heap leaching test of a Greek low-grade nickel oxide ore, aiming at verifying, at a large scale, the amenability of Greek laterites to heap leaching by the HELLAS (Heap Leaching LAteriteS) process, developed at the National Technical University of Athens for the first time worldwide and patented by some of the authors as early as in 1991. The test was conducted at the site of Aghios Ioannis mine of G.M.M.S.A. LARCO in 2006–2008 and was financed and supervised by the Institute of Geology and Mineral Exploration (I.G.M.E). The ore sample, 800 t, was from the “Triada” deposit of LARCO, in Euboea, and contained 0.73% nickel, 0.06% cobalt, 35.6% iron and 15% silicon. The ore was ground to −18 mm and the leaching agent was 2N (100 g/L) sulphuric acid solution. The nickel and cobalt recoveries obtained at the time of termination but not completion of the test, after four leaching cycles and 114 days of irrigation, were 60 and 36%, respectively. The corresponding nickel and cobalt concentrations in the produced leach liquor were 3.4 and 0.17 g/L, respectively. The value of the ratio Fe/Ni in the leach liquor was 10/1, much lower than the value 45/1 in the ore, thus showing the selectivity of the leaching of nickel over iron in the Greek ores by the above method. The consumption of sulphuric acid was 66 kg H2SO4/kg Ni recovered. The preliminary feasibility study, that followed the test, confirmed the economic viability of the integrated HELLAS process for the low-grade nickel oxide ores of Greece.
1. Introduction
An innovative integrated hydrometallurgical process for the efficient and economic nickel and cobalt extraction from Greek low-grade nickel oxide ores has been developed and patented as a result of many years of work at the Laboratory of Metallurgy of the National Technical University of Athens, Greece.
The “Heap Leach LAteriteS” (HELLAS) process comprises heap leaching of the ore with dilute sulphuric acid at ambient temperature, purification of the leach liquor by chemical precipitation at atmospheric pressure and recovery of nickel and cobalt from the purified leach liquor either by solvent extraction and electrowinning or by chemical precipitation [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24].
A research project, financed by the Institute of Geology and Mineral Exploration (I.G.M.E.) in the period 2006–2008, was undertaken to verify the leaching stage of the HELLAS process at a pilot plant scale. The test was conducted at the site of Aghios Ioannis mine of the General Mining and Metallurgical S.A. LARCO of Greece, which acted as an earthwork contractor of I.G.M.E. under the instructions of the scientific team.
2. Experimental Work
2.1. Materials
The ore used for the experiment was from the “Triada” deposit of LARCO and is very representative of the Greek laterites. The chemical analysis of the ore is shown in Table 1 and its particle size analysis in Table 2.

Table 1.
Chemical analysis of the ore.

Table 2.
Particle size analysis of the ore.
The ore was mineralogically analysed using optical microscopy, X-ray diffraction and electron microprobe analysis. The main mineralogical constituents of the ore were found to be haematite, quartz and chlorite. Chromite and illite were present in lesser amounts. Small amounts of calcite and dolomite were also determined. The chemical compositions of chlorite, illite, haematite and chromite were determined by microprobe analysis. The analysis clearly showed that the main nickeliferous mineral in the ore was chlorite, rich in iron. Based on the chemical analysis of the ore and the chemical composition of the various mineralogical phases, the percent weight of each mineral was calculated and is presented in Table 3.

Table 3.
Mineralogical composition of the “Triada” ore.
From the above data, nickel was found to be distributed 69% in chlorite, 25.4% in haematite and 5.6% in illite. Iron was distributed 89% in haematite, 8% in chlorite, 1.7% in illite and 1.3% in chromite.
2.2. Heap Leaching Prodecure
First, 800 t of ore were stacked into a heap, 3.0 m high. The impermeable pad underneath, from bottom to top, consisted of:
- (a)
- a layer of clay material, 30 cm thick;
- (b)
- a layer of fine silica sand, 5 cm thick;
- (c)
- a HDPE liner, 2 mm thick;
- (d)
- a layer of coarse (5 mm–40 mm) waste ore, 33 cm thick.
The heap leach installation also included two ponds, of around 1500 m3 each, for the pregnant solution and the barren solution, properly lined with HDPE geomembrane, and two tanks, of 21 m3 volume each, for the storage of concentrated sulphuric acid. Drip irrigation was used. Scheme 1 and Scheme 2 show the pre-semi-industrial installation of the heap.

Scheme 1.
General view of the heap leach pilot-plant.

Scheme 2.
View of the heap during operation.
Prior to placement onto the pad, the ore was sprayed with water in the bucket of a loader to raise total moisture from 8% (natural moisture) to about 10% and achieve a rough agglomeration by rocking the bucket.
The sulphuric acid solution, of a pre-determined concentration and at a pre-determined flowrate, was pumped from the pond and distributed uniformly on the top surface of the heap. After the whole volume of the leach solution had percolated through the heap, thus completing one full leach cycle, its free acid concentration was measured and adjusted to the initial pre-determined value. It was then recirculated to the top of the heap for another leach cycle to start. A number of leach cycles was performed aiming at achieving the maximum nickel extraction.
The experimental conditions were:
- (a)
- Sulphuric acid concentration in the leach solution: 2 N;
- (b)
- Leach solution volume to ore weight ratio (S/O): 1 m3/t;
- (c)
- Leach solution flowrate: 500 L/m2 × day.
3. Results

Table 4.
Analysis of the pregnant solution and percent extraction of metal cations.

Table 5.
Analysis of the pregnant solution and percent extraction of metal cations.
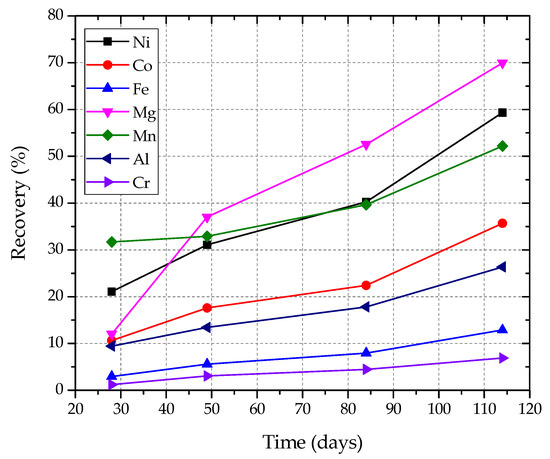
Figure 1.
Nickel and cobalt recoveries and other cations dissolution with time during leaching.
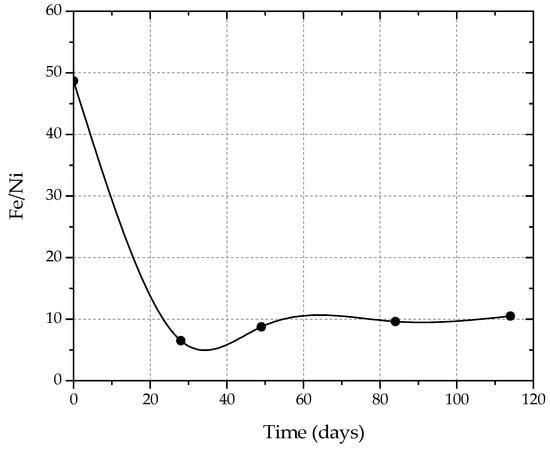
Figure 2.
Fe/Ni ratio in the pregnant solution with time during leaching.
4. Discussion
After four leaching cycles, nickel and cobalt recoveries reached about 60 and 36%, correspondingly, in 114 days (3.8 months). Higher recoveries might possibly have been obtained if the trial had not been terminated before completion, due to a late start and project time expiry. The fact that equilibrium had not been achieved is noticeable also in Figure 1.
Besides, a high proportion of nickel, 25.4% in the laterite sample, occurred in the lattice of haematite, which does not dissolve under the intentionally mild leach conditions of the HELLAS process. Additionally, the data from the mineralogical analyses of both the ore and the leach residue, unfortunately not included here due to space limitations, show that the haematitic pissolites, present in the ore, also contained numerous inclusions of chlorite, which is the main nickeliferous and the most leachable mineral of the Greek laterites.
The much lower (compared to nickel) cobalt recovery was attributed to the lower kinetics of the cobalt leaching reaction because of the very low content of the metal in the laterite ore.
It can be seen that the highest extractions obtained were those for magnesium, nickel and manganese. Their leach rate curves were similar and close to each other as all three metals occur mainly in the same mineralogical phases, i.e., chlorite and illite.
Co-dissolution values of aluminium, iron and chromium were all much lower than those of magnesium, nickel and manganese, with their curves lying further down in the figure. It should be stressed, however, that the mineralogical analyses have shown that the above metals were also leached out of the same mineralogical phases (chlorite and illite) as magnesium, nickel, manganese and cobalt. The reason why their leach rate curves lie apart from the nickel curve is due to the fact that these metals occur in a number of different minerals of the laterite and their percent extractions were calculated on the basis of their total contents in the ore and not on their contents in the leachable sheet-silicate minerals.
The apparent selectivity of nickel to iron resulted in the ratio of Fe/Ni in the pregnant solution being approximately 10.5/1, compared to 49/1 in the ore.
The consumption of sulphuric acid was measured at around 66 kg H2SO4/kg Ni extracted. This increased, relative to previous experiments, consumption was attributed to:
- (a)
- high calcite content of the waste ore layer of the pad;
- (b)
- high iron content (~23%) of the chlorite in the specific ore sample.
5. Conclusions
- This pre-semi-industrial trial confirmed the conclusion of the authors from previous lab-scale and pilot-scale column leaching tests that the Greek low-grade laterites can efficiently be leached by dilute sulphuric acid according to the HELLAS process of heap leaching. The pre-feasibility study, performed both together and independently by the inventors of the process and I.G.M.E. [23,24] but not included in this paper due to space limitations, has shown that the integrated hydrometallurgical HELLAS process is also economical.
- The main characteristic and advantage of the HELLAS process is the selectivity of nickel and cobalt dissolution over iron at the proposed leaching conditions.
- Even though the ore was not properly agglomerated, due to the lack of suitable equipment, the permeability of the heap was good and was not disturbed during the trial. However, proper agglomeration would allow higher solution flowrates, and therefore, shorter leach times.
- The most important factor that affects the leachability of a laterite ore by the heap leaching technique, the recoveries of nickel and cobalt, the co-dissolution of all other metal cations and, consequently, the sulphuric acid consumption is the mineralogy of the ore. The higher the percent weight of the phyllosilicate minerals, and particularly that of chlorite, in the ore and the higher the content of nickel and cobalt in the above minerals, the higher the recoveries of the useful metals. Additionally, the lower the content of iron and magnesium in the phyllosilicate minerals of the ore, the lower the sulphuric acid consumption.
- Given that almost all laterite deposits in Greece have a mineralogical analysis favourable for the application of the HELLAS process, its industrial use will allow the exploitation of low-grade deposits that cannot be treated economically by pyrometallurgical methods, thus prolonging the lifetime of the Greek nickel industry.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This statement can be excluded because the study did not report any data.
Acknowledgments
The authors are grateful to I.G.M.E. for financing the project through the operational program “Competitiveness” (2000–2006).
References
- Agatzini, S.; Dimaki, D. Nickel and Cobalt Recovery from Low-Grade Nickel Oxide Ores by the Technique of Heap Leaching Using Dilute Sulphuric Acid at Ambient Temperature. Greek Patent No. 1001555, 31 May 1991. [Google Scholar]
- Agatzini-Leonardou, S.; Dimaki, D. Method for Extraction of Nickel and/or Cobalt from Nickel and/or Cobalt Oxide Ores by Heap Leaching with a Dilute Sulphuric Acid Solution, Prepared from Sea Water, at Ambient Temperature. Greek Patent No. 1003569, 23 April 2001. [Google Scholar]
- Agatzini-Leonardou, S.; Oustadakis, P.; Zafiratos, J. Process for the Removal of Aluminium and/or Chromium from Nickel and/or Cobalt Sulphate Solutions at Atmospheric Pressure. Greek Patent No. 1003419, 19 April 1999. [Google Scholar]
- Agatzini, S.; Karidakis, T. Production of a Magnesium Hydroxide—Containing Mixture Suitable for Use as a Filler in Polymers and as an Additive in Cement. Greek Patent No. 1003693, 18 July 2000. [Google Scholar]
- Agatzini-Leonardou, S.; Dimaki, D. Heap leaching of poor nickel laterites by sulphuric acid at ambient temperature. In Proceedings of the International Symposium “Hydrometallurgy ’94”, Cambridge, UK, 11–15 July 1994; pp. 193–208. [Google Scholar]
- Agatzini-Leonardou, S.; Tsakiridis, P.; Neou-Syngouna, P.; Kaselouri, B. Nickel-cobalt separation in sulphate solutions by solvent extraction with Cyanex 272. In Proceedings of the 1st National Congress of Chemical Engineering, Patra, Greece, 29–31 May 1997; pp. 283–288. [Google Scholar]
- Agatzini-Leonardou, S.; Dimaki, D.; Mposkos, E. Extraction of nickel and cobalt from Greek low-grade nickel oxide ores by heap leaching. In Proceedings of the Nickel-Cobalt 97 International Symposium, Sudbury, ON, Canada, 17–20 August 1997; Cooper, W.C., Mihailov, I., Eds.; Volume 1, pp. 489–503. [Google Scholar]
- Agatzini-Leonardou, S.; Tsakiridis, P. Simultaneous cobalt and magnesium extraction from nickel sulphate solutions. In Proceedings of the XX International Mineral Processing Congress, Aachen, Germany, 21–26 September 1997; Hoberg, H., von Blottnitz, H., Eds.; Volume 4, pp. 271–283. [Google Scholar]
- Agatzini-Leonardou, S.; Neou-Syngouna, P.; Karidakis, T. Removal of magnesium from sulphate solutions by chemical precipitation. Trans. Inst. Min. Metall. 1998, 107, 87–164. [Google Scholar]
- Agatzini, S.; Tsakiridis, P. Cobalt magnesium separation by solvent extraction with bis(2,4,4-trimethyl-pentyl) monothiophosphinic acid. In Proceedings of the XXI International Mineral Processing Congress, Rome, Italy, 23–27 July 2000; Massacci, P., Ed.; Volume A, pp. A6/22–A6/32. [Google Scholar]
- Agatzini-Leonardou, S.; Oustadakis, P. Removal of aluminum and chromium from leach liquors produced by sulphuric acid leaching of nickel oxide ores. In Proceedings of the IX Balkan Mineral Processing Congress, Istanbul, Turkey, 11–13 September 2001; Onal, G., Atak, S., Guney, A., Celik, M., Yuce, A., Eds.; pp. 525–529. [Google Scholar]
- Tsakiridis, P.E.; Agatzini, S.L. Simultaneous solvent extraction of cobalt and nickel in the presence of manganese and magnesium from sulphate solutions by Cyanex 301. Hydrometallurgy 2004, 72, 269–278. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Agatzini, S.L. Process for the recovery of cobalt and nickel in the presence of magnesium and calcium from sulphate solutions by Versatic 10 and Cyanex 272. Miner. Eng. 2004, 17, 535–543. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Agatzini-Leonardou, S.L. Process for the recovery of cobalt and nickel in the presence of magnesium from sulphate solutions by Cyanex 272 and Cyanex 302. Miner. Eng. 2004, 17, 913–923. [Google Scholar] [CrossRef]
- Agatzini-Leonardou, S.; Zafiratos, J.G. Beneficiation of a Greek serpentinic nickeliferous ore—Part II: Sulphuric Acid Heap Leaching. Hydrometallurgy 2004, 74, 267–275. [Google Scholar] [CrossRef]
- Katsioti, M.; Boura, P.; Agatzini, S.; Tsakiridis, P.E.; Oustadakis, P. Use of jarosite/alunite precipitate as a substitute for gypsum in Portland cement. Cem. Concr. Compos. 2005, 27, 3–9. [Google Scholar] [CrossRef]
- Agatzini, S.; Karidakis, T.; Tsakiridis, P.E. Use of Gypsum/Brucite Mixed Precipitate Instead of Gypsum in Portland Cement. J. Chem. Technol. Biotechnol. 2005, 80, 320–324. [Google Scholar] [CrossRef]
- Karidakis, T.; Agatzini-Leonardou, S.; Neou-Syngouna, P. Removal of magnesium from nickel laterite leach liquors by chemical precipitation using calcium hydroxide and the potential use of the precipitate as a filler material. Hydrometallurgy 2005, 76, 105–114. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Agatzini, S.; Oustadakis, P.; Katsioti, M.; Mauridou, E. Examination of the Jarosite-Alunite Precipitate Addition in the Raw Meal for the Production of Portland Cement Clinker. Cem. Concr. Res. 2005, 35, 2066–2073. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Agatzini-Leonardou, S. Simultaneous solvent extraction of cobalt and magnesium in the presence of nickel from sulphate solutions by Ionquest 801. J. Chem. Technol. Biotechnol. 2005, 80, 1236–1243. [Google Scholar] [CrossRef]
- Oustadakis, P.; Agatzini-Leonardou, S.; Tsakiridis, P.E. Nickel and cobalt co-precipitation from sulphate leach liquor by MgO pulp as neutralizing agent. Miner. Eng. 2005, 19, 1204–1211. [Google Scholar] [CrossRef]
- Oustadakis, P.; Agatzini-Leonardou, S.; Tsakiridis, P.E. Bulk precipitation of nickel and cobalt from sulphate leach liquor by CaO pulp. Miner. Process Extr. Metall. Rev. 2007, 116, 245–250. [Google Scholar] [CrossRef]
- Agatzini-Leonardou, S.; Leonardos, M.; Oustadakis, P.; Tsakiridis, P.E. Prefeasibility study of the integrated HELLAS process. In Novel Technologies-Techniques for Evaluation and Exploitation of Metallic Minerals; Task 14; I.G.M.E.: Athens, Greece, November 2008. (In Greek) [Google Scholar]
- Panteli, Z. Prefeasibility study for a hydrometallurgical plant with annual capacity of 10000t Ni. In Novel Technologies-Techniques for Evaluation and Exploitation of Metallic Minerals; Task 13; I.G.M.E.: Athens, Greece, June 2009; Available online: https://libraryigme.openabekt.gr/el/document/5c76a5a0294801ba0df9c06c (accessed on 25 November 2021). (In Greek)
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).