CO2-Mineralised Nesquehonite: A New “Green” Building Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Nesquehonite
2.2. Synthesis of the New “Green” Building Material
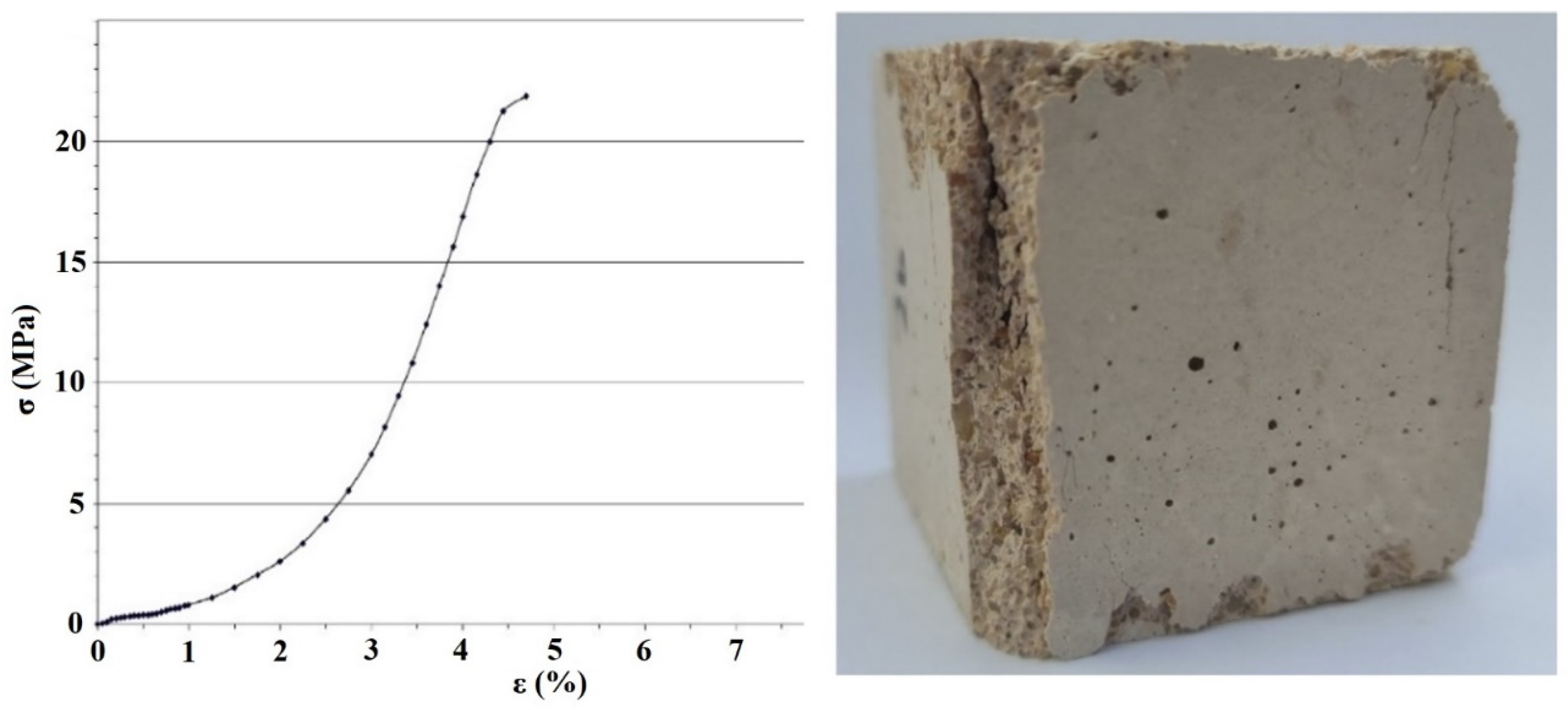
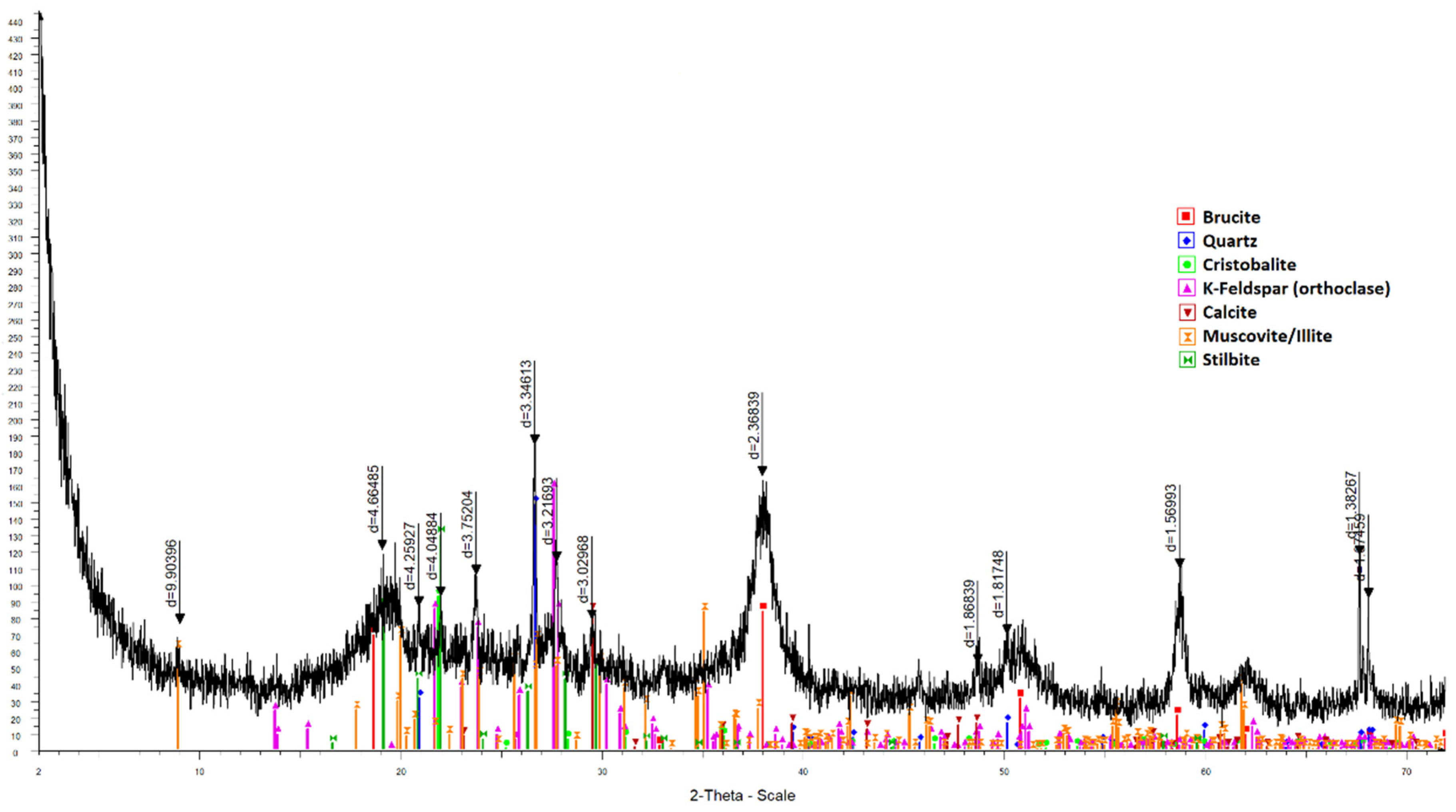
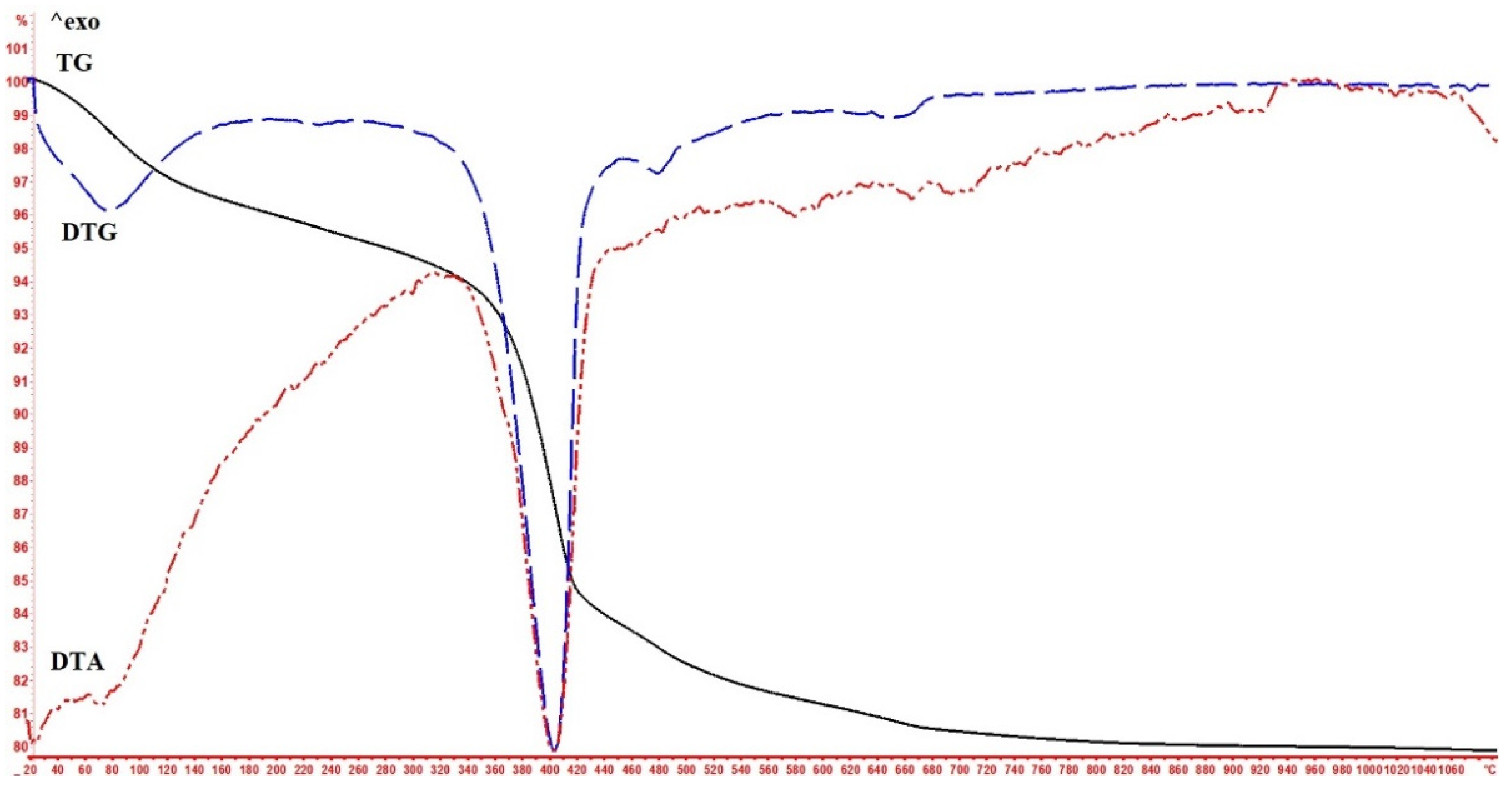
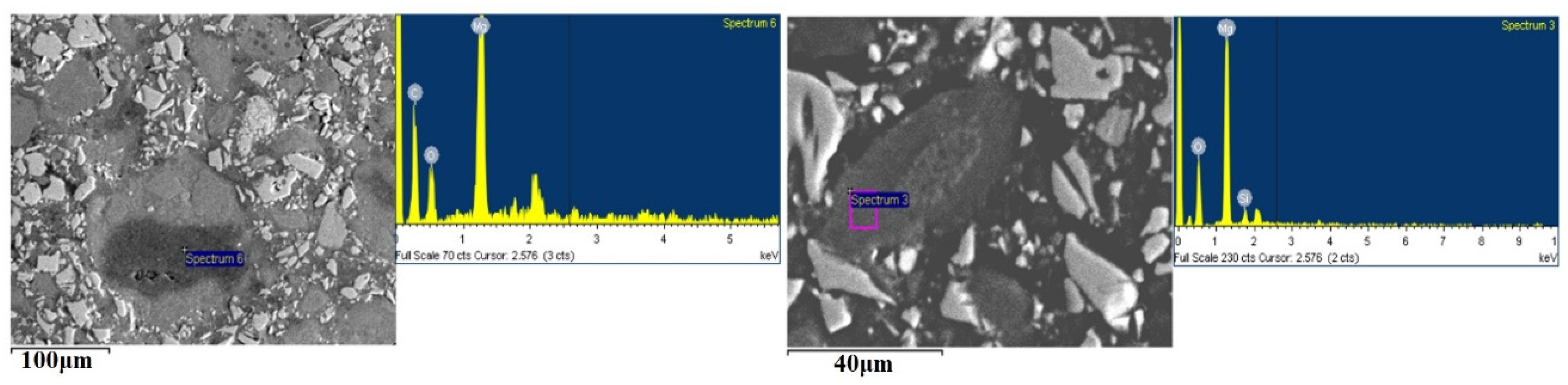
3. Results
4. Discussion
5. Conclusions
Author Contributions
References
- Gerdemann, S.J.; O’Connor, W.K.; Dahlin, D.C.; Penner, L.R.; Rush, H. Ex Situ Aqueous Mineral Carbonation. Environ. Sci. Technol. 2007, 41, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Gislason, S.R.; Oelkers, E.H. Carbon Storage in Basalt. Science 2014, 344, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Lackner, K.S.; Wendt, C.; Butts, D.P.; Joyce, E.L.; Sharps, D.H. Carbon Dioxide Disposal in Carbonate Mineral. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Matter, J.M.; Kelemen, P.B. Permanent Storage of Carbon Dioxide in Geological Reservoirs by Mineral Carbonation. Nat. Geosci. 2009, 2, 837–841. [Google Scholar] [CrossRef]
- Matter, J.M.; Stute, M.; Snaebjornsdottir, S.O.; Oelkers, E.H.; Gislason, S.R.; Aradottir, E.S.; Sigfusson, B.; Gunnarsson, I.; Sigurdardottir, H.; Gunnlaugsson, E.; et al. Rapid Carbon Mineralization for Permanent Disposal of Anthropogenic Carbon Dioxide Emissions. Science 2016, 352, 1312–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michael, K.; Neal, P.R.; Allinson, G.; Ennis-King, J.; Hou, W.; Paterson, L.; Sharma, S.; Aiken, T. Injection Strategies for Large-Scale CO2 Storage Sites. Energy Procedia 2011, 4, 4267–4274. [Google Scholar] [CrossRef] [Green Version]
- Galvez-Martos, J.L.; Morrison, J.; Jauffret, G.; Elsarrag, E.; AlHorr, Y.; Imbabi, M.S.; Glasser, F.P. Environmental Assessment of Aqueous Alkaline Absorption of Carbon Dioxide and Its Use to Produce a Construction Material. Resour. Conserv. Recycl. 2016, 107, 129–141. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Fagerlund, J.; Björklöf, T.; Mäkelä, M.; Eklund, O. Carbon Dioxide Mineralisation and Integration with Flue Gas Desulphurisation Applied to a Modern Coal-Fired Power Plant. In Proceedings of the ECOS2012, Perugia, Italy, 26–29 June 2012. [Google Scholar]
- Ferrini, V.; De Vito, C.; Mignardi, S. Synthesis of Nesquehonite by Reaction of Gaseous CO2 with Mg Chloride Solution: Its Potential Role in the Sequestration of Carbon Dioxide. J. Hazard. Mater. 2009, 168, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Glasser, F.P.; Jauffret, G.; Morrison, J.; Galvez-Martos, J.-L.; Patterson, N.; Imbabi, M.S.-E. Sequestering CO2 by Mineralization into Useful Nesquehonite-Based Products. Front. Energy Res. 2016, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Vito, C.D.; Ferrini, V.; Mignardi, S.; Cagnetti, M.; Leccese, F. Ex Situ CO2 Mineralization via Nesquehonite: A First Attempt for an Industrial Application. In Proceedings of the 2012 11th International Conference on Environment and Electrical Engineering, Venice, Italy, 18–25 May 2012; pp. 323–327. [Google Scholar]
- Ballirano, P.; De Vito, C.; Ferrini, V.; Mignardi, S. The Thermal Behaviour and Structural Stability of Nesquehonite, MgCO3·3H2O, Evaluated by in Situ Laboratory Parallel-Beam X-ray Powder Diffraction: New Constraints on CO2 Sequestration within Minerals. J. Hazard. Mater. 2010, 178, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Ballirano, P.; De Vito, C.; Mignardi, S.; Ferrini, V. Phase Transitions in the MgCO2H2O System and the Thermal Decomposition of Dypingite, Mg5(CO3)4(OH)2·5H2O: Implications for Geosequestration of Carbon Dioxide. Chem. Geol. 2013, 340, 59–67. [Google Scholar] [CrossRef]
- Madlool, N.A.; Saidur, R.; Hossain, M.S.; Rahim, N.A. A Critical Review on Energy Use and Savings in the Cement Industries. Renew. Sustain. Energy Rev. 2011, 15, 2042–2060. [Google Scholar] [CrossRef]
- Chatterjee, A.K. Chemistry and Engineering of the Clinkerization Process—Incremental Advances and Lack of Breakthroughs. Cem. Concr. Res. 2011, 41, 624–641. [Google Scholar] [CrossRef]
- Ruan, S.; Unluer, C. Comparative Life Cycle Assessment of Reactive MgO and Portland Cement Production. J. Clean. Prod. 2016, 137, 258–273. [Google Scholar] [CrossRef]
- Unluer, C.; Al-Tabbaa, A. Impact of Hydrated Magnesium Carbonate Additives on the Carbonation of Reactive MgO Cements. Cem. Concr. Res. 2013, 54, 87–97. [Google Scholar] [CrossRef]
- Vandeperre, L.J.; Al-Tabbaa, A. Accelerated Carbonation of Reactive MgO Cements. Adv. Cem. Res. 2007, 19, 67–79. [Google Scholar] [CrossRef]
- Panesar, D.K.; Mo, L. Properties of Binary and Ternary Reactive MgO Mortar Blends Subjected to CO2 Curing. Cem. Concr. Compos. 2013, 38, 40–49. [Google Scholar] [CrossRef]
- Liska, M.; Al-Tabbaa, A. Performance of Magnesia Cements in Porous Blocks in Acid and Magnesium Environments. Adv. Cem. Res. 2012, 24, 221–232. [Google Scholar] [CrossRef]
- Skliros, V.; Tsakiridis, P.; Perraki, M. A Combined Raman, Fourier Transform Infrared, and X-ray Diffraction Study of Thermally Treated Nesquehonite. J. Raman Spectrosc. 2020, 51, 1445–1453. [Google Scholar] [CrossRef]
- Stephan, G.W.; MacGillavry, C.H. The Crystal Structure of Nesquehonite, MgCO3. 3H2O. Acta Crystallogr. B Struct. Sci. 1972, 28, 1031–1033. [Google Scholar] [CrossRef]
- Yang, N.; Tran, M.H.; Scott, A.; Dhakal, R.P.; Watson, M.; Shi, C.J. Properties of Magnesium Based Cements. In Proceedings of the Wellington: New Zealand Concrete Industry Conference, Wellington, New Zealand, 12–14 October 2017. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kastrinakis, A.; Skliros, V.; Tsakiridis, P.; Perraki, M. CO2-Mineralised Nesquehonite: A New “Green” Building Material. Mater. Proc. 2021, 5, 60. https://doi.org/10.3390/materproc2021005060
Kastrinakis A, Skliros V, Tsakiridis P, Perraki M. CO2-Mineralised Nesquehonite: A New “Green” Building Material. Materials Proceedings. 2021; 5(1):60. https://doi.org/10.3390/materproc2021005060
Chicago/Turabian StyleKastrinakis, Anthony, Vasilios Skliros, Petros Tsakiridis, and Maria Perraki. 2021. "CO2-Mineralised Nesquehonite: A New “Green” Building Material" Materials Proceedings 5, no. 1: 60. https://doi.org/10.3390/materproc2021005060
APA StyleKastrinakis, A., Skliros, V., Tsakiridis, P., & Perraki, M. (2021). CO2-Mineralised Nesquehonite: A New “Green” Building Material. Materials Proceedings, 5(1), 60. https://doi.org/10.3390/materproc2021005060






