Production of Metallic Titanium by Electrowinning in Molten Salts of Titanium Oxycarbide Anode †
Abstract
:1. Introduction
2. Experimental
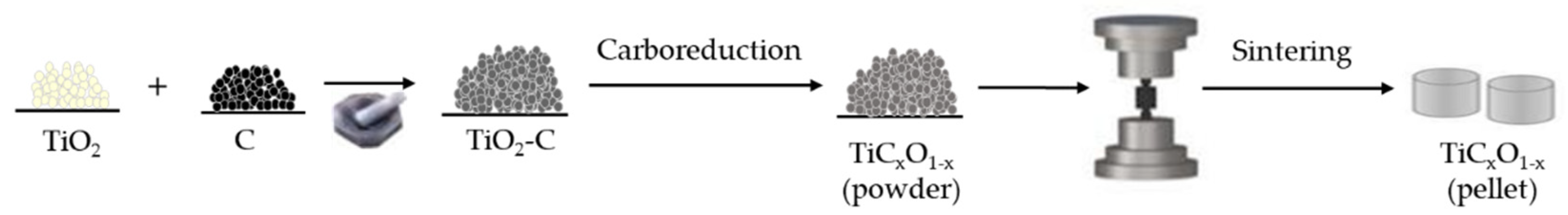
2.1. Preparation of Titanium Oxycarbide Anode
2.2. Electrolysis Process
2.3. Electrodes
2.4. Electrochemical Techniques Used
2.5. Materials Characterization
3. Results and Discussion
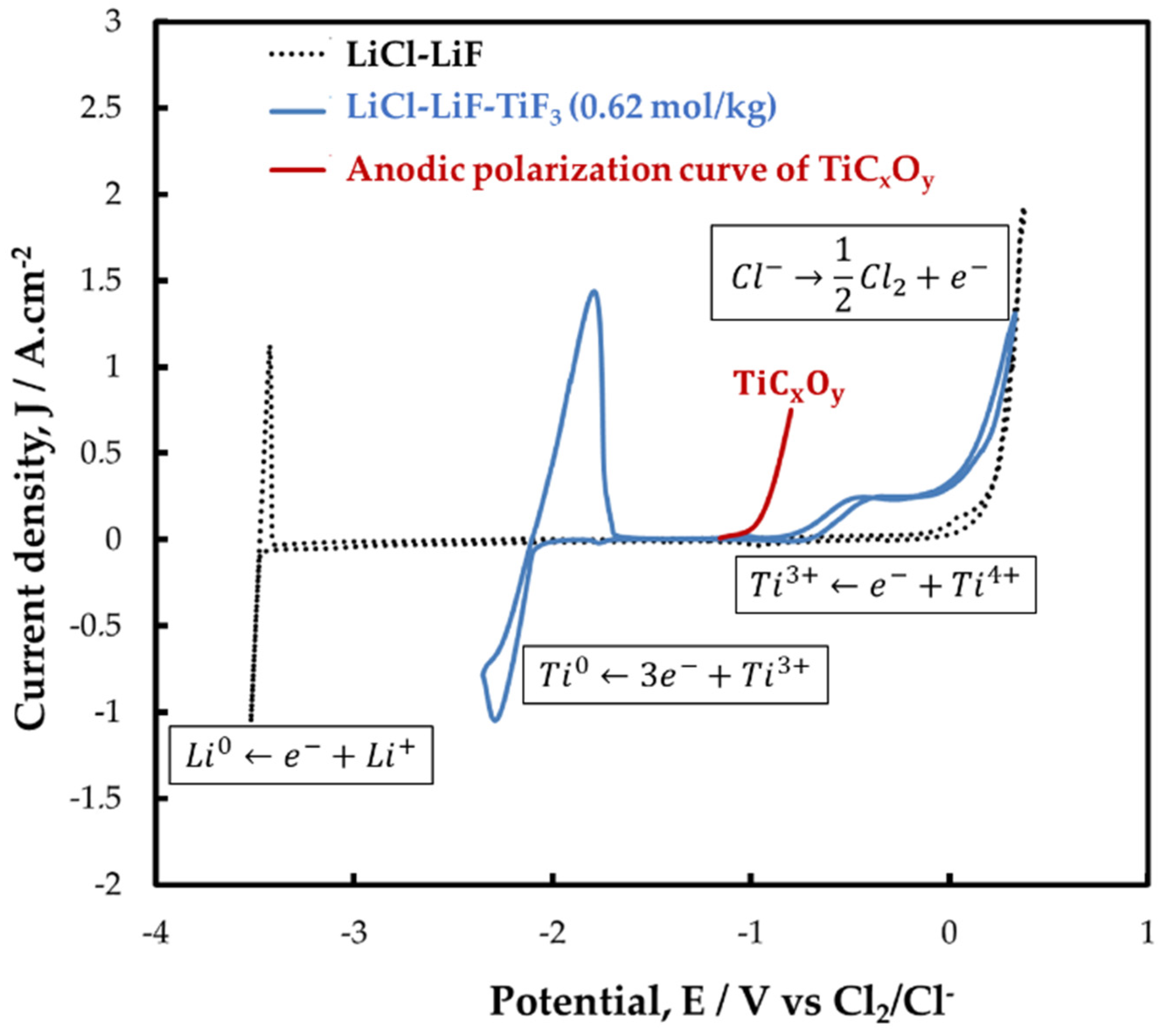
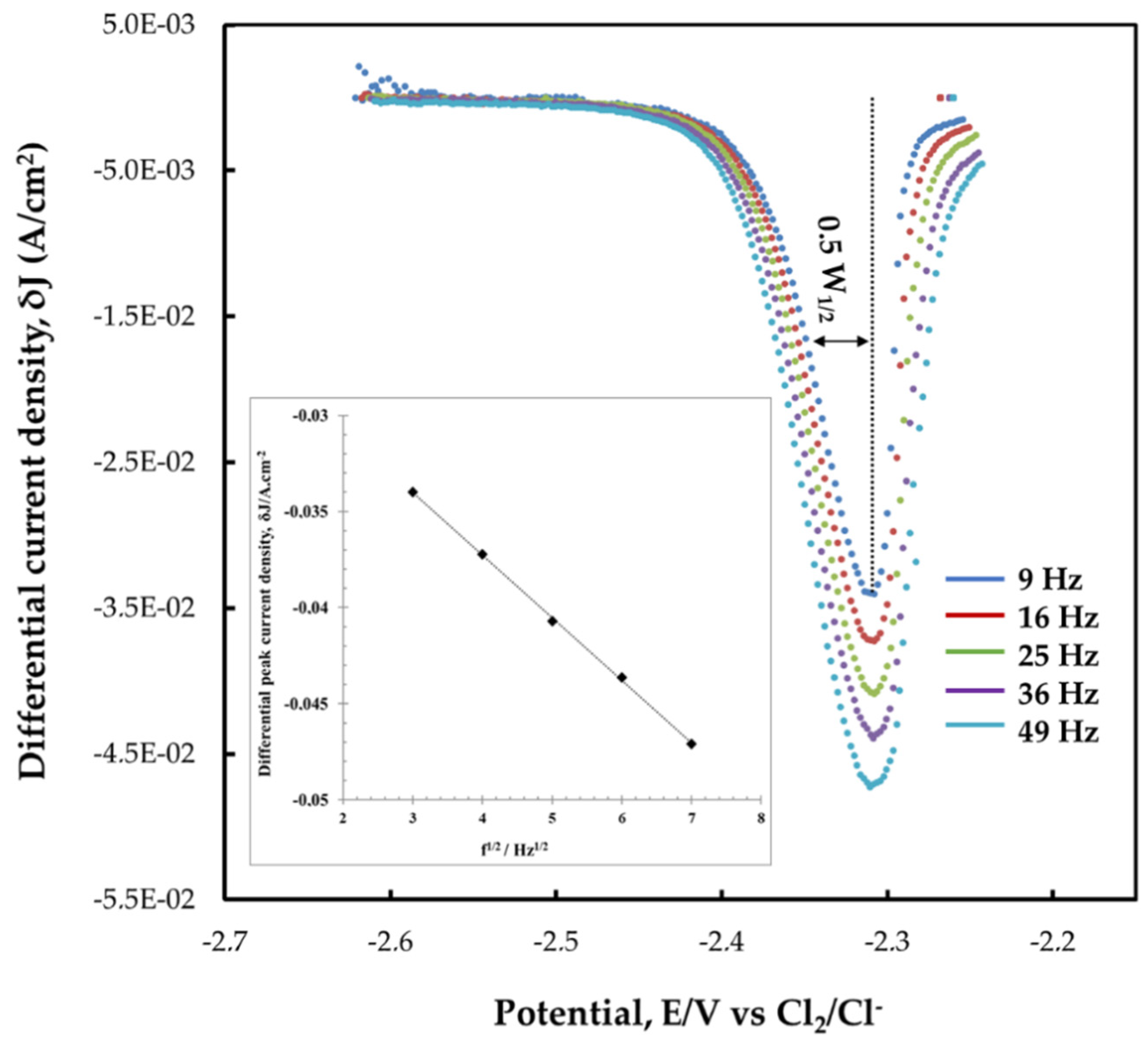
3.1. Electrochemical Behavior of Titanium Ions
3.1.1. Cyclic Voltammetry (CV)
3.1.2. Square Wave Voltammetry (SWV)
3.1.3. Titanium Oxycarbide Anodic Dissolution
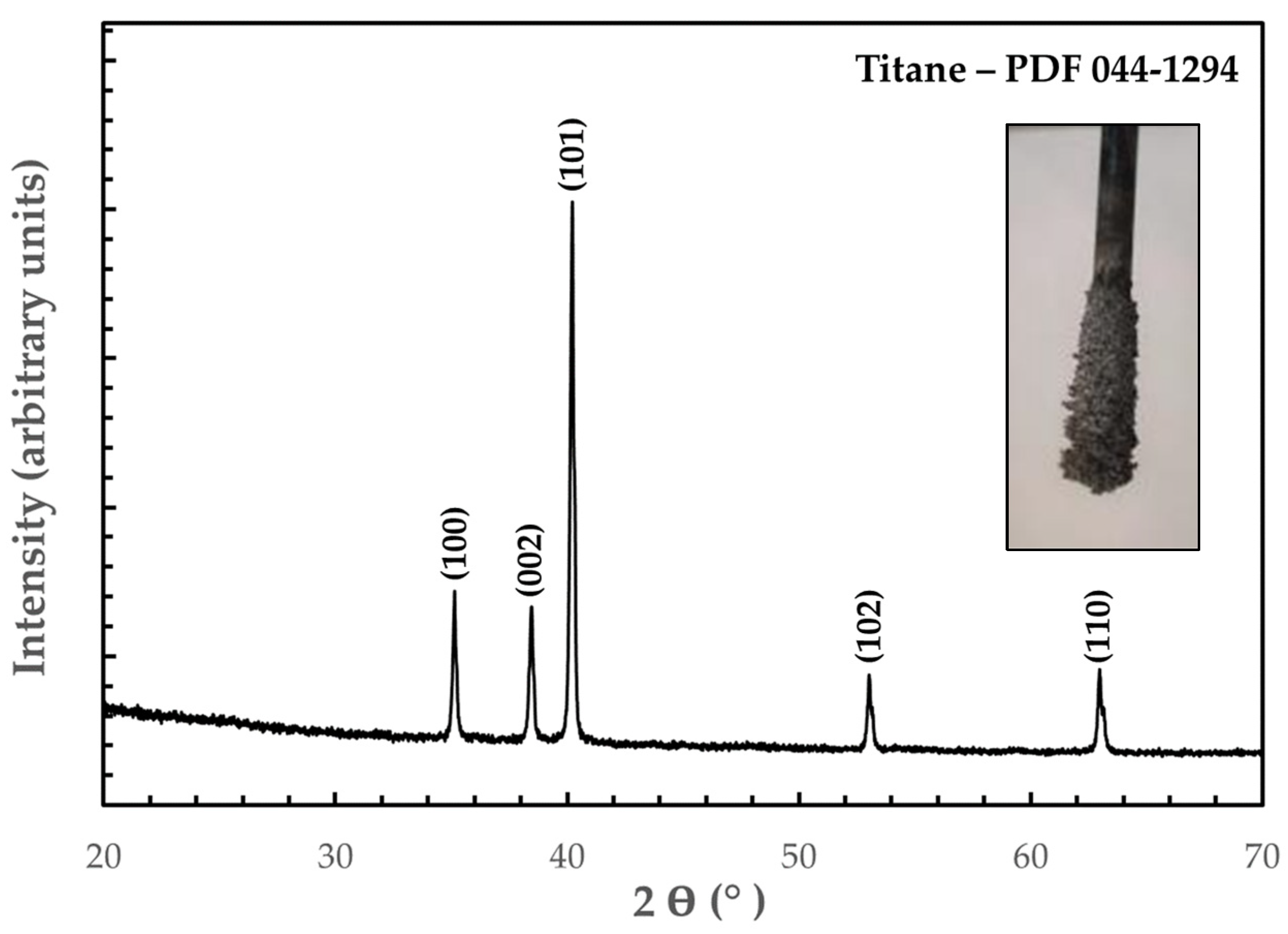
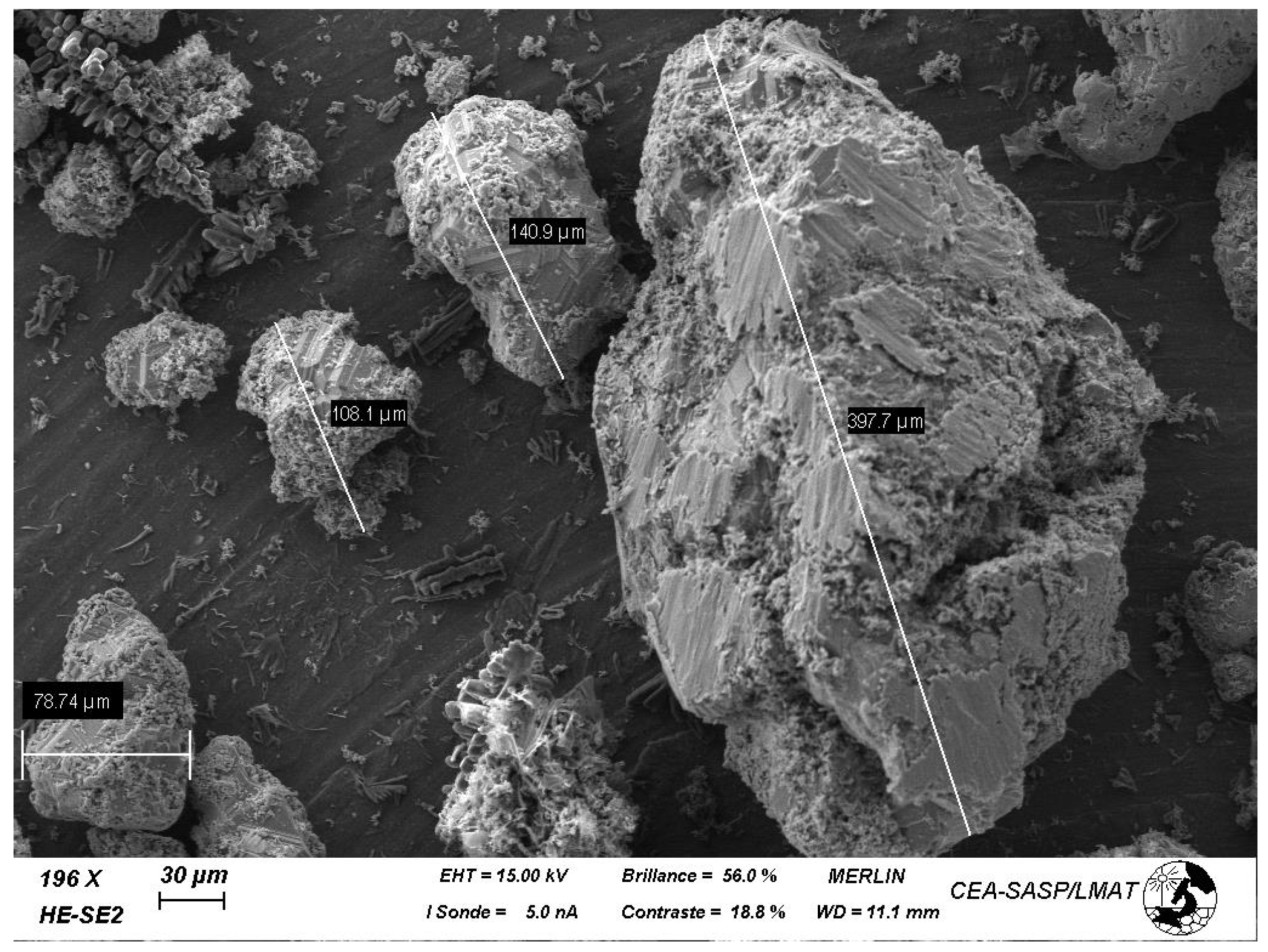
3.2. Electrodeposition of Titanium
4. Conclusions
References
- Yves COMBRES. Propriétés du Titane et de ses Alliages. Techniques de l’ingénieur, Réf.: M4780 V1. 10 March 2010. Available online: https://www.techniques-ingenieur.fr/res/pdf/encyclopedia/42357210-m4780.pdf (accessed on 4 December 2021).
- Kroll, W.; Fink, C.G.; Summers, D.B. The Production of Ductile Titanium. Trans. Electrochem. Soc. 1940, 78, 35–649. [Google Scholar] [CrossRef]
- van Vuuren, D.S. A critical evaluation of processes to produce primary titanium. J. S. Afr. Inst. Min. Metal. 2009, 109, 455–461. [Google Scholar]
- Marco, V.; Ginatta, G.T.T. Titanium Electrowinning. In Proceedings of the International Symposium on Ionic Liquids in Honour of Marcelle Gaune-Escard. Carry le Rouet, France, 27–28 June 2003; pp. 3–4. [Google Scholar]
- Chen, G.; Fray, D.J.; Farthing, T.W. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Nat. Cell Biol. 2000, 407, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Suzuki, R.O. A new concept for producing Ti sponge: Calciothermic reduction. J. Miner. Metals Mater. Soc. 2002, 54, 59–61. [Google Scholar] [CrossRef]
- Mohandas, K.S. Direct electrochemical conversion of metal oxides to metal by molten salt electrolysis: A review. Miner. Process. Extr. Met. 2013, 122, 195–212. [Google Scholar] [CrossRef]
- Bertolini, M.; Shaw, L.; England, L.; Rao, K.; Deane, J.; Collins, J. The FFC Cambridge Process for Production of Low Cost Titanium and Titanium Powders. Key Eng. Mater. 2010, 436, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Fray, D.J.; Jiao, S. Treatment of Titanium Ores. Chinuka Limited. GB 2472496. 28 July 2010. Available online: https://www.mysciencework.com/patent/download/treatment-titanium-ores-EP2462251B1/EP2462251B1 (accessed on 4 December 2021).
- Ning, X.; Xiao, J.; Jiao, S.; Zhu, H. Anodic dissolution of titanium oxycarbide TiCxO1−x with different O/C ratio. J. Electrochem. Soc. 2019, 166, E22–E28. [Google Scholar] [CrossRef]
- Popov, B.N.; Kimbl e, M.C.; White, R.E.; Wendt, H. Electrochemical behaviour of titanium(II) and titaniun(III) compounds in molten lithium chloride/potassium chloride eutectic melts. J. Appl. Electrochem. 1991, 21, 351–357. [Google Scholar] [CrossRef]
- Zhu, H. Rare Earth Metal Production by Molten Salt Electrolysis. In Encyclopedia of Applied Electrochemistry; Springer: New York, NY, USA, 2014; pp. 1765–1772. [Google Scholar]
- Head, R.B. Electrolytic Production of Sintered Titanium from Titanium Tetrachloride at a Contact Cathode. J. Electrochem. Soc. 1961, 108, 806. [Google Scholar] [CrossRef]
- Haarberg, G.M.; Rolland, W.; Sterten, A.; Thonstad, J. Electrodeposition of titanium from chloride melts. J. Appl. Electrochem. 1993, 23, 217–224. [Google Scholar] [CrossRef]
- Song, J.; Wang, Q.; Zhu, X.; Hou, J.; Jiao, S.; Zhu, H. The Influence of Fluoride Anion on the Equilibrium between Titanium Ions and Electrodeposition of Titanium in Molten Fluoride–Chloride Salt. Mater. Trans. 2014, 55, 1299–1303. [Google Scholar] [CrossRef] [Green Version]
- Norikawa, Y.; Yasuda, K.; Nohira, T. Electrodeposition of Titanium in a Water-Soluble KF–KCl Molten Salt. Mater. Trans. 2017, 58, 390–394. [Google Scholar] [CrossRef] [Green Version]
- Réjasse, F. Study of the Reactivity of Group Ivb Metallic Dioxides in the Presence of Carbon by a (Micro)-Structural Approach: Application to the Thermodynamic Modelling of Ternary Phase Diag. Ph.D. Thesis, University of Limoges, Limoges, France, 2 December 2015; pp. 125–243. [Google Scholar]
- Gendre, M.; Maître, A.; Trolliard, G. Synthesis of zirconium oxycarbide (ZrCxOy) powders: Influence of stoichiometry on densification kinetics during spark plasma sintering and on mechanical properties. J. Eur. Ceram. Soc. 2011, 31, 2377–2385. [Google Scholar] [CrossRef]
- Geran, S.; Chamelot, P.; Serp, J.; Gibilaro, M.; Massot, L. Electrochemistry of Uranium in Molten LiCl-LiF; Elsevier: Amsterdam, The Netherlands, 23 April 2020. [Google Scholar]
- Ramaley, L.; Krause, M.S. Theory of square wave voltammetry. Anal. Chem. 1969, 41, 1362–1365. [Google Scholar] [CrossRef]
- Dring, K. Challenges and Opportunities in Titanium Metal Production, Is There Still Any Interest/Need to Replace Kroll Production? Norsk Titanium AS: Oslo, Norway; pp. 12–14. Available online: https://www.okabe.iis.u-tokyo.ac.jp/workshop/090219_Workshop_SanFrancisco_for_Okabe_lab_HP/parts/doc.C.pdf (accessed on 4 November 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malek, B.; Serp, J.; Doreau, F.; Miguirditchian, M.; Vandenhende, M.; Pradeilles, N.; Lepetitcorps, Y.; Maitre, A. Production of Metallic Titanium by Electrowinning in Molten Salts of Titanium Oxycarbide Anode. Mater. Proc. 2021, 5, 63. https://doi.org/10.3390/materproc2021005063
Malek B, Serp J, Doreau F, Miguirditchian M, Vandenhende M, Pradeilles N, Lepetitcorps Y, Maitre A. Production of Metallic Titanium by Electrowinning in Molten Salts of Titanium Oxycarbide Anode. Materials Proceedings. 2021; 5(1):63. https://doi.org/10.3390/materproc2021005063
Chicago/Turabian StyleMalek, Btissem, Jerome Serp, Franck Doreau, Manuel Miguirditchian, Marion Vandenhende, Nicolas Pradeilles, Yann Lepetitcorps, and Alexandre Maitre. 2021. "Production of Metallic Titanium by Electrowinning in Molten Salts of Titanium Oxycarbide Anode" Materials Proceedings 5, no. 1: 63. https://doi.org/10.3390/materproc2021005063
APA StyleMalek, B., Serp, J., Doreau, F., Miguirditchian, M., Vandenhende, M., Pradeilles, N., Lepetitcorps, Y., & Maitre, A. (2021). Production of Metallic Titanium by Electrowinning in Molten Salts of Titanium Oxycarbide Anode. Materials Proceedings, 5(1), 63. https://doi.org/10.3390/materproc2021005063






