ΣIDERWIN—A New Route for Iron Production †
Abstract
:1. Introduction
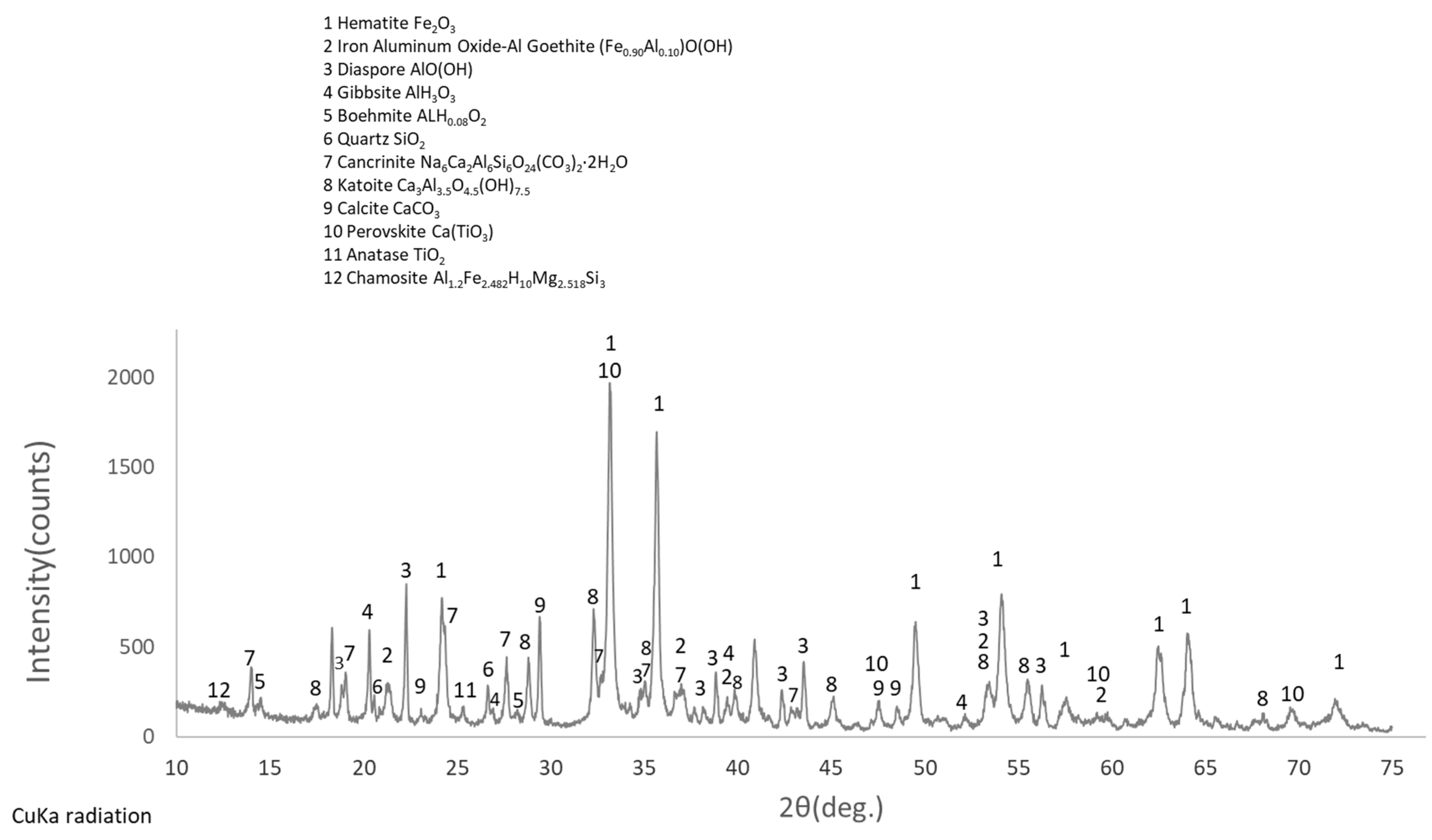
2. Materials and Methods
2.1. Materials
2.2. Experimental Set-Up and Parameters
2.3. Faradaic Efficiency
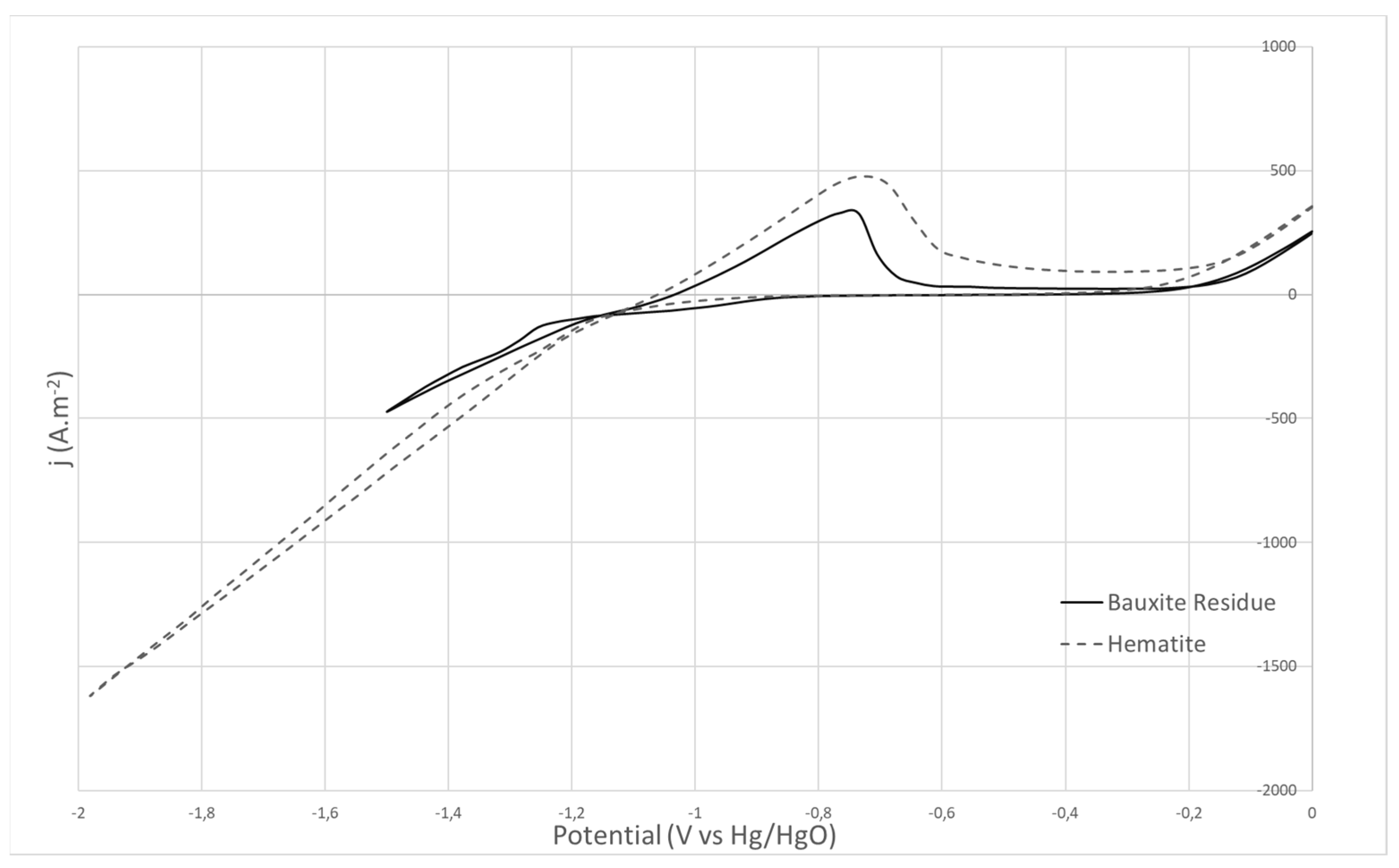
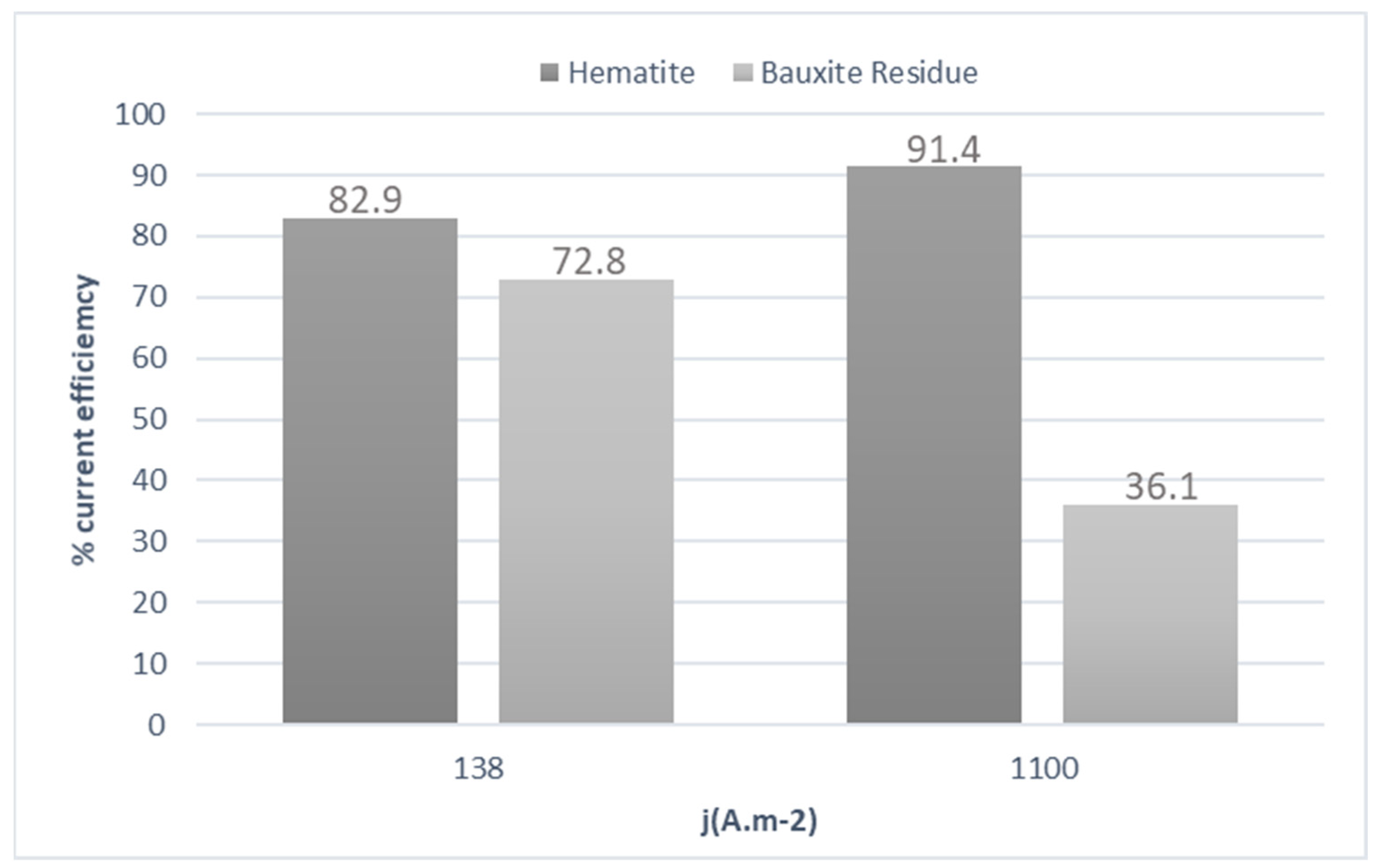
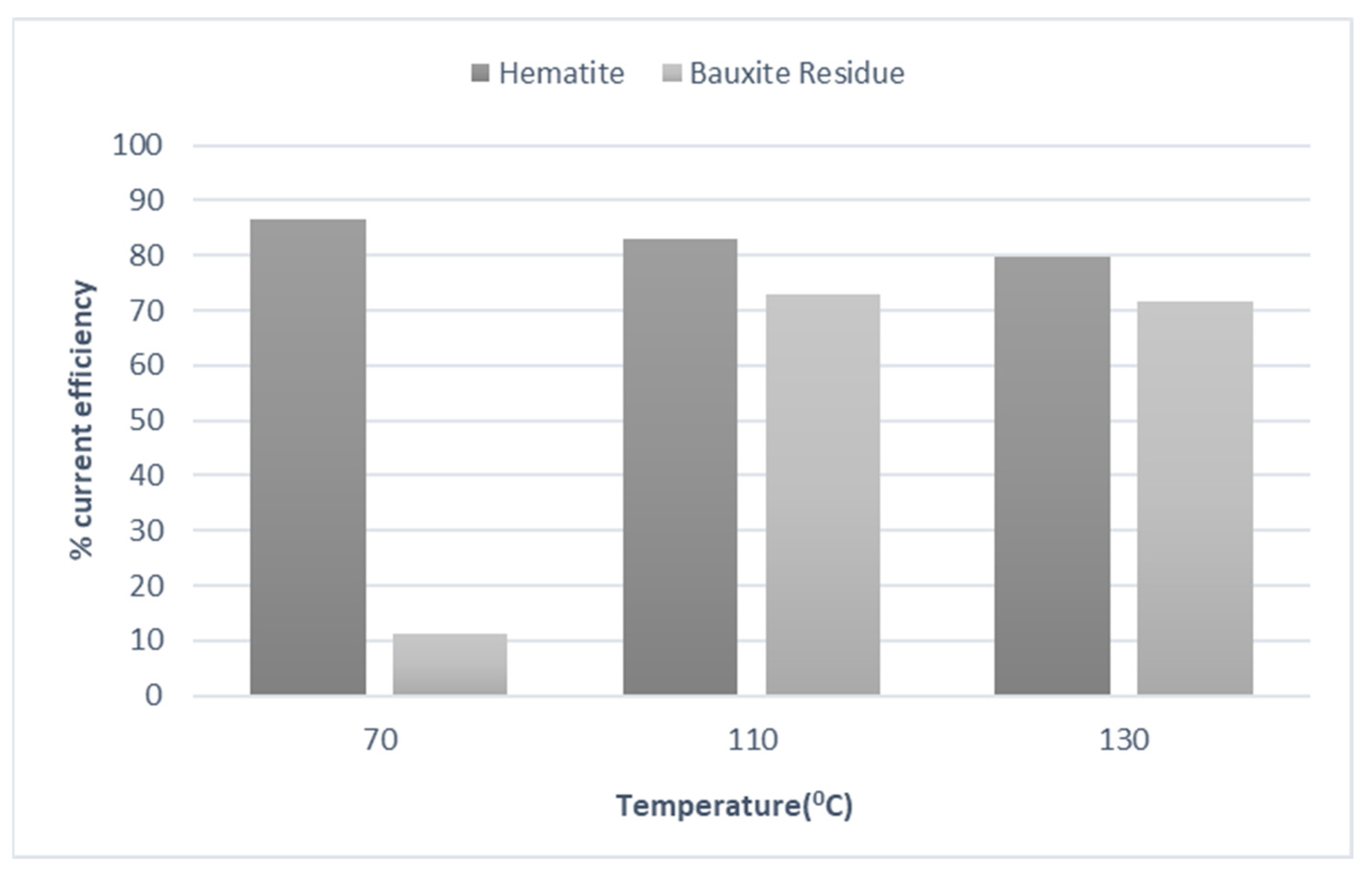
3. Results and Discussion
3.1. Cyclic Voltammetry
3.2. Galvanostatic Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Steel Association. Climate Change and the Production of Iron and Steel; World Steel Association: Brussels, Belgium, 2021. [Google Scholar]
- Yuan, B.; Haarberg, G.M. Electrowinning of Iron in Aqueous Alkaline Solution Using Rotating Disk Electrode. Revue de Métallurgie 2009, 106, 455–459. [Google Scholar] [CrossRef]
- Junjie, Y. Progress and Future of Breakthrough Low-carbon Steelmaking Technology (ULCOS) of EU. Int. J. Miner. Process. Extr. Metall. 2018, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Allanore, A.; Lavelaine, H.; Valentin, G.; Birat, J.P.; Delcroix, P.; Lapicque, F. Observation and modeling of the reduction of hematite particles to metal in alkaline solution by electrolysis. Electrochim. Acta 2010, 55, 4007–4013. [Google Scholar] [CrossRef]
- Allanore, A.; Lavelaine, H.; Birat, J.P.; Valentin, G.; Lapicque, F. Experimental investigation of cell design for the electrolysis of iron oxide suspensions in alkaline electrolyte. J. Appl. Electrochem. 2010, 40, 1957–1966. [Google Scholar] [CrossRef]
- Allanore, A.; Lavelaine, H.; Valentin, G.; Birat, J.P.; Lapicque, F. Iron Metal Production by Bulk Electrolysis of Iron Ore Particles in Aqueous Media. J. Electrochem. Soc. 2008, 155. [Google Scholar] [CrossRef]
- Lavelaine, H.; Allanore, A. Optimized Design of an Iron Electrowinning Cell. Revue de Métallurgie 2009, 106, 460–471. [Google Scholar] [CrossRef]
- Balomenos, E.; Davris, P.; Pontikes, Y.; Panias, D. Mud2Metal: Lessons Learned on the Path for Complete Utilization of Bauxite Residue Through Industrial Symbiosis. J. Sustain. Metall. 2017, 3, 551–560. [Google Scholar] [CrossRef]
- Koutsoupa, S.; Koutalidi, S.; Bourbos, E.; Balomenos, E.; Panias, D. Electrolytic iron production from alkaline bauxite residue slurries at low temperatures. Johns. Matthey Technol. Rev. 2021. [Google Scholar] [CrossRef]
| Metal Oxides | Percentage (%) |
|---|---|
| Fe2O3 | 39.19 |
| Al2O3 | 23.81 |
| SiO | 7.68 |
| CaO | 8.07 |
| Na2O | 3.44 |
| TiO2 | 5.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koutsoupa, S.; Koutalidi, S.; Balomenos, E.; Panias, D. ΣIDERWIN—A New Route for Iron Production. Mater. Proc. 2021, 5, 58. https://doi.org/10.3390/materproc2021005058
Koutsoupa S, Koutalidi S, Balomenos E, Panias D. ΣIDERWIN—A New Route for Iron Production. Materials Proceedings. 2021; 5(1):58. https://doi.org/10.3390/materproc2021005058
Chicago/Turabian StyleKoutsoupa, Sevasti, Stavroula Koutalidi, Efthymios Balomenos, and Dimitrios Panias. 2021. "ΣIDERWIN—A New Route for Iron Production" Materials Proceedings 5, no. 1: 58. https://doi.org/10.3390/materproc2021005058
APA StyleKoutsoupa, S., Koutalidi, S., Balomenos, E., & Panias, D. (2021). ΣIDERWIN—A New Route for Iron Production. Materials Proceedings, 5(1), 58. https://doi.org/10.3390/materproc2021005058






