Preliminary Characterization of Three Metallurgical Bauxite Residue Samples †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
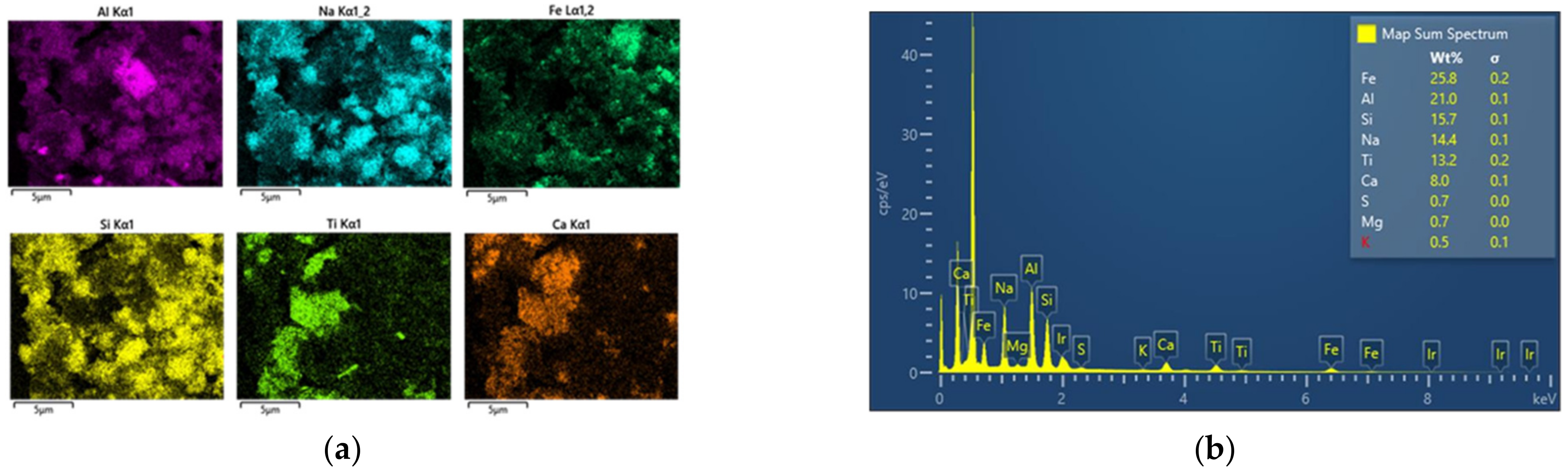
3.1. Chemical Composition
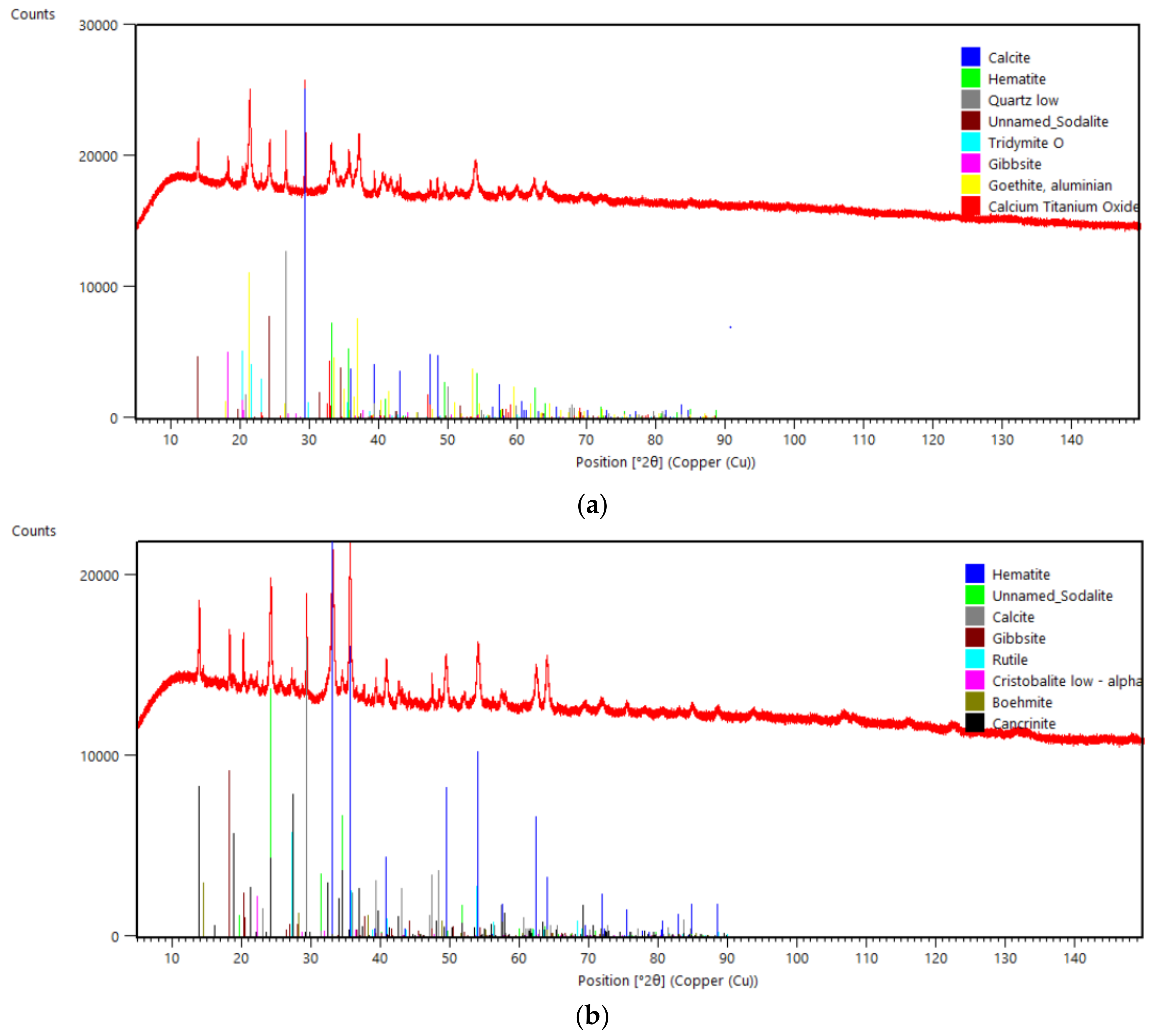
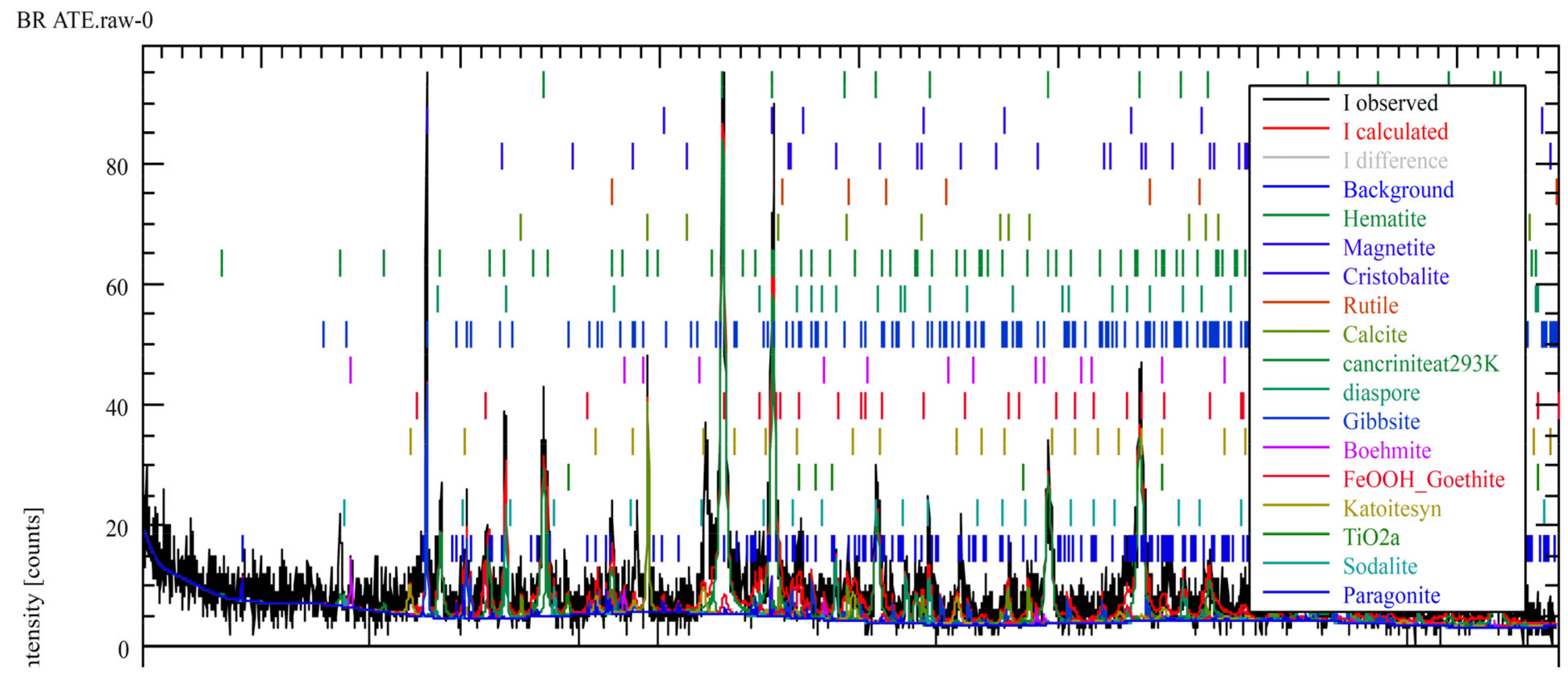
3.2. Bulk Solid Phase Characterization
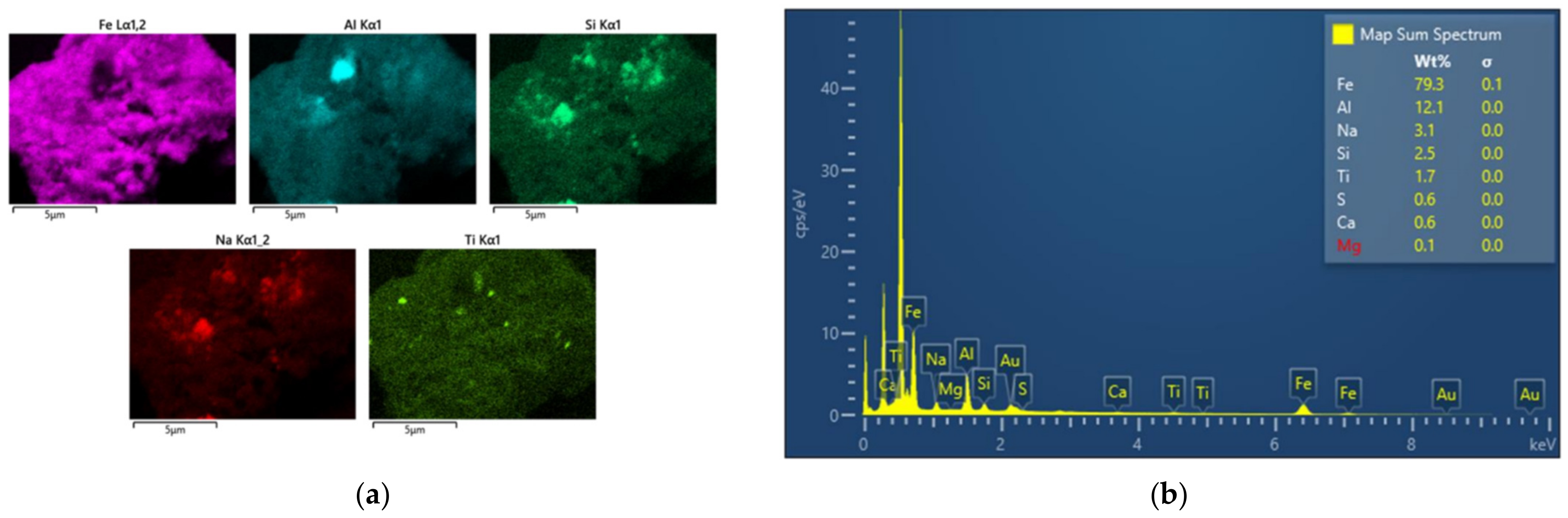
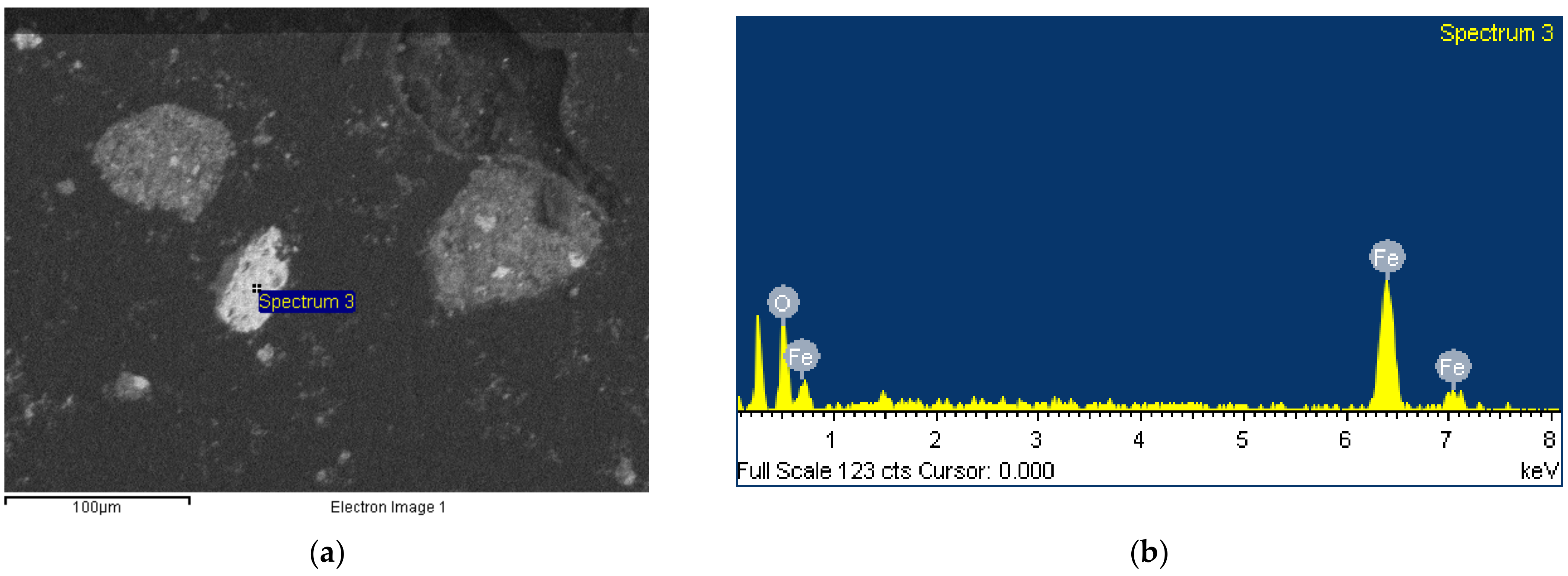
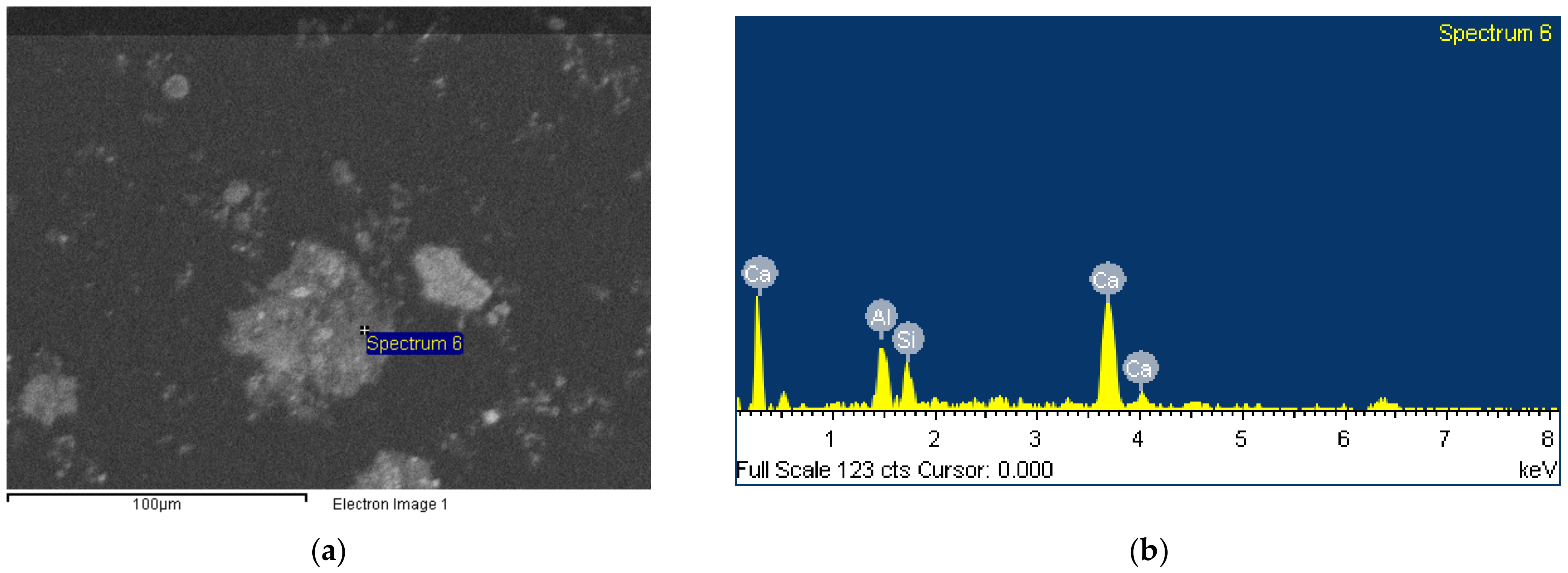
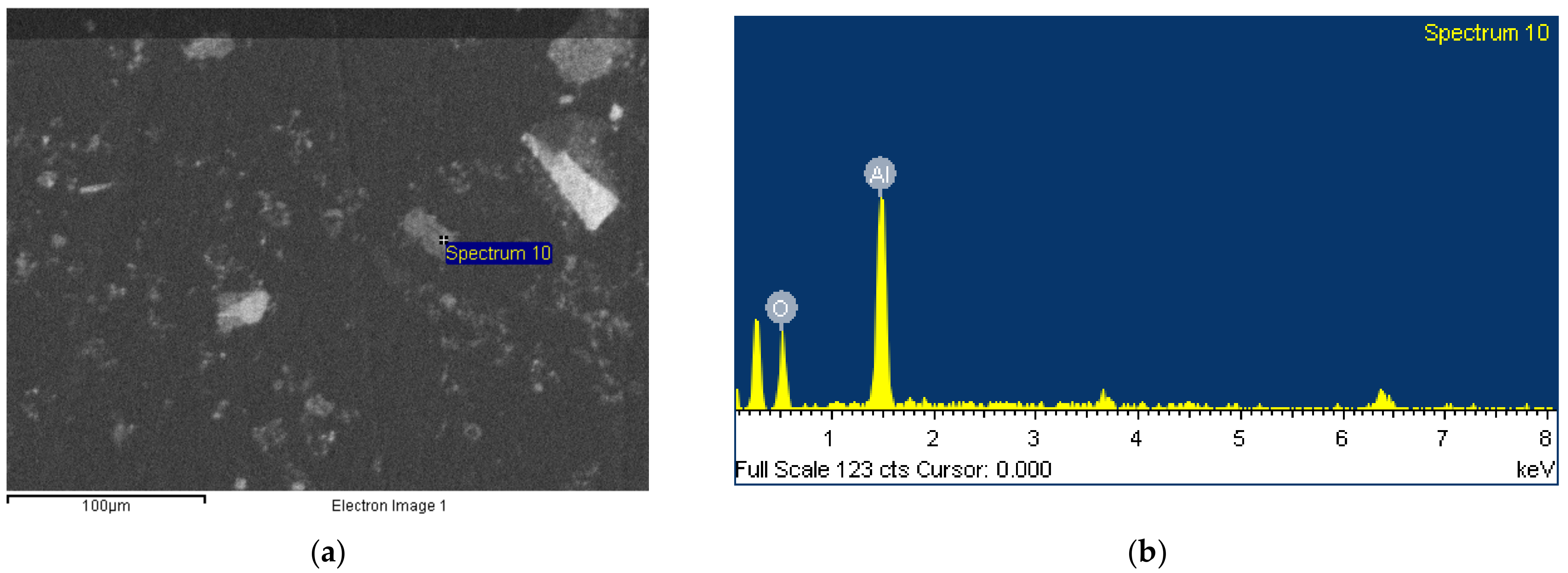
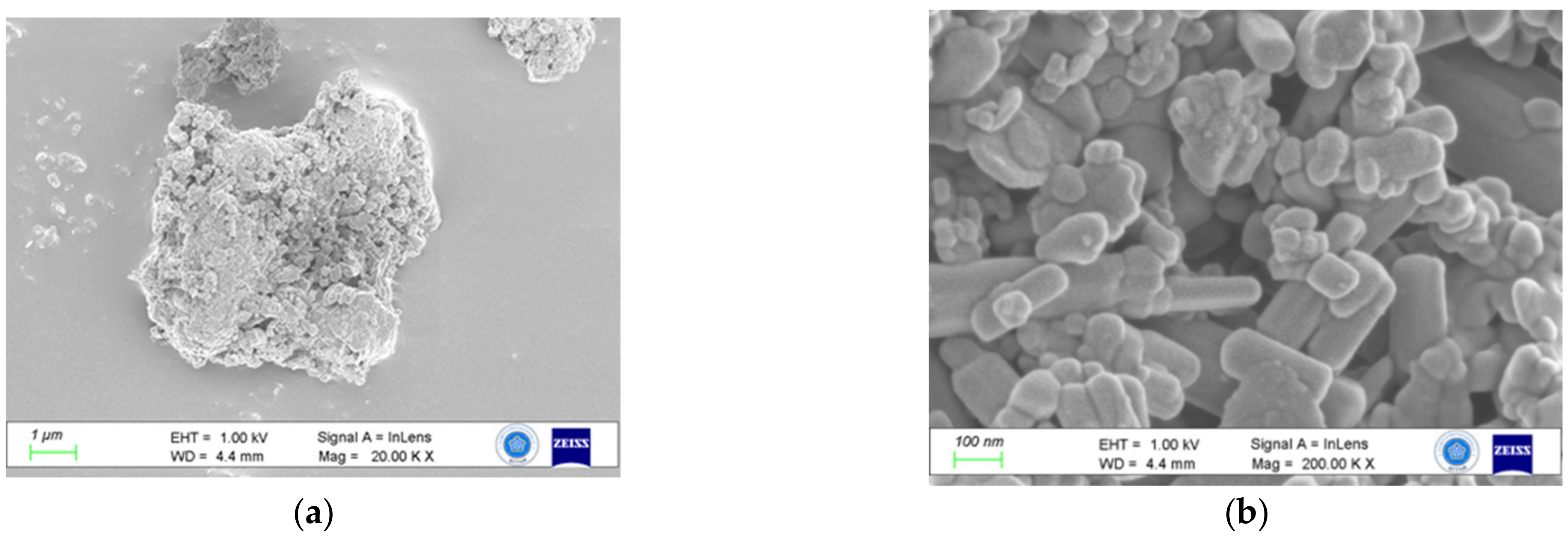
3.3. Morphology
4. Conclusions
- Regarding partial chemical composition and preliminary solid phase characterization of BR samples, in general, small differences were observed, which can be attributed to the different origins of the ores used, as well as to the different processing conditions applied in the Bayer process. More significant deviations were identified for sodium and iron content. Major REEs and Sc scandium were detected in all cases. Their concentrations will be determined through ICP-MS on a following study.
- In the Romanian sample (ALUM), iron was mainly found in the form of goethite, while in the Greek (AoG) and the Turkish (ETI) samples, iron was structured in hematite.
- Despite the tiny size of BR particles, usually more than one phase coexist on a single grain, thus burdening the purity of samples that can be produced after the implementation of a physical separation procedure.
Funding
Informed Consent Statement
Conflicts of Interest
References
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Milačič, R.; Zuliani, T.; Ščančar, J. Environmental impact of toxic elements in red mud studied by fractionation and speciation procedures. Sci. Total Environ. 2012, 426, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Heal, K.V.; Friesl-Hanl, W. The use of red mud as an immobiliser for metal/metalloid-contaminated soil: A review. J. Hazard. Mater. 2017, 325, 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vind, J.; Malfliet, A.; Blanpain, B.; Tsakiridis, P.; Tkaczyk, A.; Vassiliadou, V.; Panias, D. Rare earth element phases in bauxite residue. Minerals 2018, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Vind, J.; Malfliet, A.; Bonomi, C.; Paiste, P.; Sajó, I.E.; Blanpain, B.; Tkaczyk, A.; Vassiliadou, V.; Panias, D. Modes of occurrences of scandium in Greek bauxite and bauxite residue. Miner. Eng. 2018, 123, 35–48. [Google Scholar] [CrossRef]
- Ujaczki, E.; Zimmermann, Y.; Gasser, C.; Molnár, M.; Feigl, V.; Lenz, M. Red mud as secondary source for critical raw materials-purification of rare earth elements by liquid/liquid extraction. J. Chem. Technol. Biotechnol. 2017, 92, 2835–2844. [Google Scholar] [CrossRef]
- Döbelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Oxide | BR ALUM (%) | BR AoG (%) | BR ETI (%) |
|---|---|---|---|
| Na2O | 5.94 | 3.94 | 15.63 |
| MgO | 0.58 | 0.18 | 0.26 |
| Al2O3 | 15.85 | 15.40 | 15.33 |
| SiO2 | 9.92 | 8.27 | 14.06 |
| K2O | 0.078 | 0.13 | 0.47 |
| CaO | 5.27 | 9.46 | 6.99 |
| TiO2 | 2.81 | 6.16 | 5.29 |
| Cr2O3 | 0.14 | 0.29 | 0.07 |
| Fe2O3 | 45.97 | 46.70 | 32.36 |
| NiO | 0.012 | 0.12 | 0.05 |
| LOI | 11.97 | 8.29 | 8.29 |
| Other | 1.41 | 1.06 | 1.20 |
| Element | BR ALUM (mg/kg) | BR AoG (mg/kg) | BR ETI (mg/kg) |
|---|---|---|---|
| La | <100 | 133.3 | 142 |
| Ce | <100 | 304.1 | 392 |
| Y | <200 | <200 | 135 |
| Nd | <200 | <200 | 178 |
| Sc | ~150 | ~150 | 76 |
| Compound Name | Chemical Formula | Content (%) | ||
|---|---|---|---|---|
| BR-ALUM | BR-AoG | BR-ETI | ||
| Goethite, aluminian | Al0.1Fe0.9OH | 46.3 | 15.63 | |
| Sodalite | Al6Na6Si6C2.4O34.32H23.04 | 18.4 | 0.7 | 20.9 |
| Hematite | Fe2O3 | 13.9 | 43.0 | 44.7 |
| Magnetite | Fe3O4 | 0.5 | ||
| Cancrinite | Al6Na7.14Si7.08O31.6H9.74 | 18.0 | 12.9 | |
| Calcite | CaCO3 | 11.8 | 6.7 | 7.9 |
| Quartz low | SiO2 | 3.4 | ||
| Gibbsite | Al(OH)3 | 3.2 | 9.0 | 6.7 |
| Paragonite | Al3NaSi3O12H2 | 3.0 | 3.5 | |
| Boehmite | AlO(OH) | 1.3 | 1.0 | |
| Diaspore | α-AlO(OH) | 9.0 | ||
| Calcium Sodium Aluminium Oxide (8.3/1.5/6/18) | Al6Ca8.25Na1.5O18 | 0.9 | ||
| Tridymite O | SiO2 | 2.9 | ||
| Cristobalite-α | SiO2 | 0.8 | ||
| Calcium Titanium Oxide | CaTiO3 | 0.3 | ||
| Rutile | TiO2 | 0.4 | 0.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulos, P.; Georgiou, M.; Oustadakis, P.; Taxiarchou, M.; Karadağ, H.; Eker, Y.; Dobra, G.; Boiangiu, A.; Demir, G.; Arslan, S.; et al. Preliminary Characterization of Three Metallurgical Bauxite Residue Samples. Mater. Proc. 2021, 5, 66. https://doi.org/10.3390/materproc2021005066
Angelopoulos P, Georgiou M, Oustadakis P, Taxiarchou M, Karadağ H, Eker Y, Dobra G, Boiangiu A, Demir G, Arslan S, et al. Preliminary Characterization of Three Metallurgical Bauxite Residue Samples. Materials Proceedings. 2021; 5(1):66. https://doi.org/10.3390/materproc2021005066
Chicago/Turabian StyleAngelopoulos, Panagiotis, Maria Georgiou, Paschalis Oustadakis, Maria Taxiarchou, Hakan Karadağ, Yasin Eker, Gheorghe Dobra, Alina Boiangiu, Gökhan Demir, Sedat Arslan, and et al. 2021. "Preliminary Characterization of Three Metallurgical Bauxite Residue Samples" Materials Proceedings 5, no. 1: 66. https://doi.org/10.3390/materproc2021005066
APA StyleAngelopoulos, P., Georgiou, M., Oustadakis, P., Taxiarchou, M., Karadağ, H., Eker, Y., Dobra, G., Boiangiu, A., Demir, G., Arslan, S., Davris, P., & Balomenos, E. (2021). Preliminary Characterization of Three Metallurgical Bauxite Residue Samples. Materials Proceedings, 5(1), 66. https://doi.org/10.3390/materproc2021005066







