How to Design the Utilization of Larger Scrap Share in Aluminum Production †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.C.; Wallington, T.J. Life-Cycle Energy and Greenhouse Gas Emission Benefits of Lightweighting in Automobiles: Review and Harmonization. Environ. Sci. Technol. 2013, 47, 6089–6097. [Google Scholar] [CrossRef] [PubMed]
- Considine, D.M.; Considine, G.D. Van Nostrand’s Scientific Encyclopedia; Springer Science & Buisiness Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Trowell, K.A.; Goroshin, S.; Frost, D.; Bergthorson, J. Aluminum and its role as a recyclable, sustainable carrier of renewable energy. Appl. Energy 2020, 275, 115112. [Google Scholar] [CrossRef]
- BDhindaw, K.; Aditya, G.S.; Mandal, A. Recycling and Downstream Processing of Aluminium Alloys for Automotive Applications. Encycl. Renew. Sustain. Mater. 2019, 1–8. [Google Scholar] [CrossRef]
- Haraldsson, J.; Johansson, M.T. Review of measures for improved energy efficiency in production-related processes in the aluminium industry—From electrolysis to recycling. Renew. Sustain. Energy Rev. 2018, 93, 525–548. [Google Scholar] [CrossRef] [Green Version]
- Ingarao, G. Manufacturing strategies for efficiency in energy and resources use: The role of metal shaping processes. J. Clean. Prod. 2017, 142, 2872–2886. [Google Scholar] [CrossRef]
- Miller, W.; Zhuang, L.; Bottema, J.; Wittebrood, A.; Smet, P.d.; Haszler, A.; Vieregge, A. Recent development in aluminium alloys for the automotive industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Gilstad, G. Life cycle assessment of secondary aluminium refining. In Light Metals 2014; Springer: Cham, Switzerland, 2013; pp. 901–906. [Google Scholar] [CrossRef]
- Andersson, J.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc and DICTRA, Computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- aluSELECT—Online Version. Available online: https://aluminium.matplus.eu/en/pub/home (accessed on 30 June 2021).
- MatWeb. Available online: www.matweb.com (accessed on 30 June 2021).
- Gülver, M.; Meydanoglu, O.; Işıksaçan, C. Softening Behavior of Direct Chill and Twin-Roll Cast AA 3105 Alloy. Light Met. 2019, 2019, 1143–1147. [Google Scholar] [CrossRef]
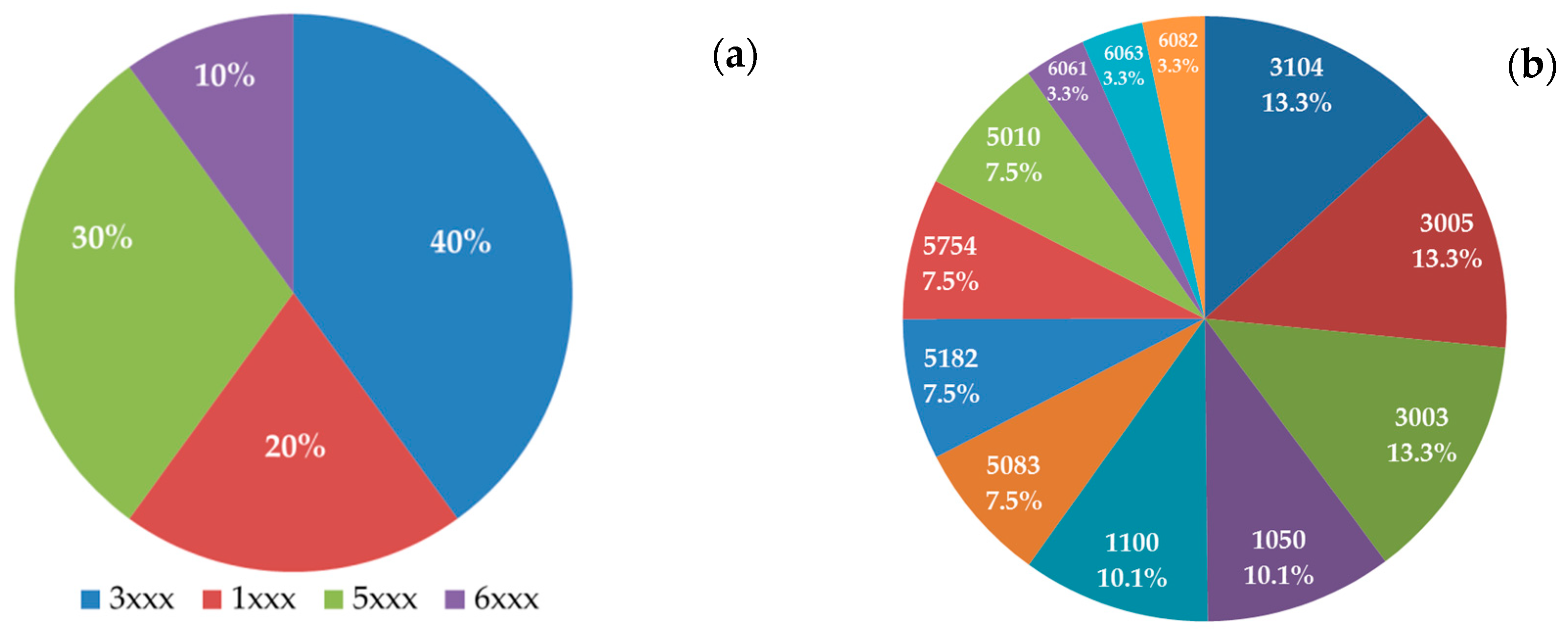
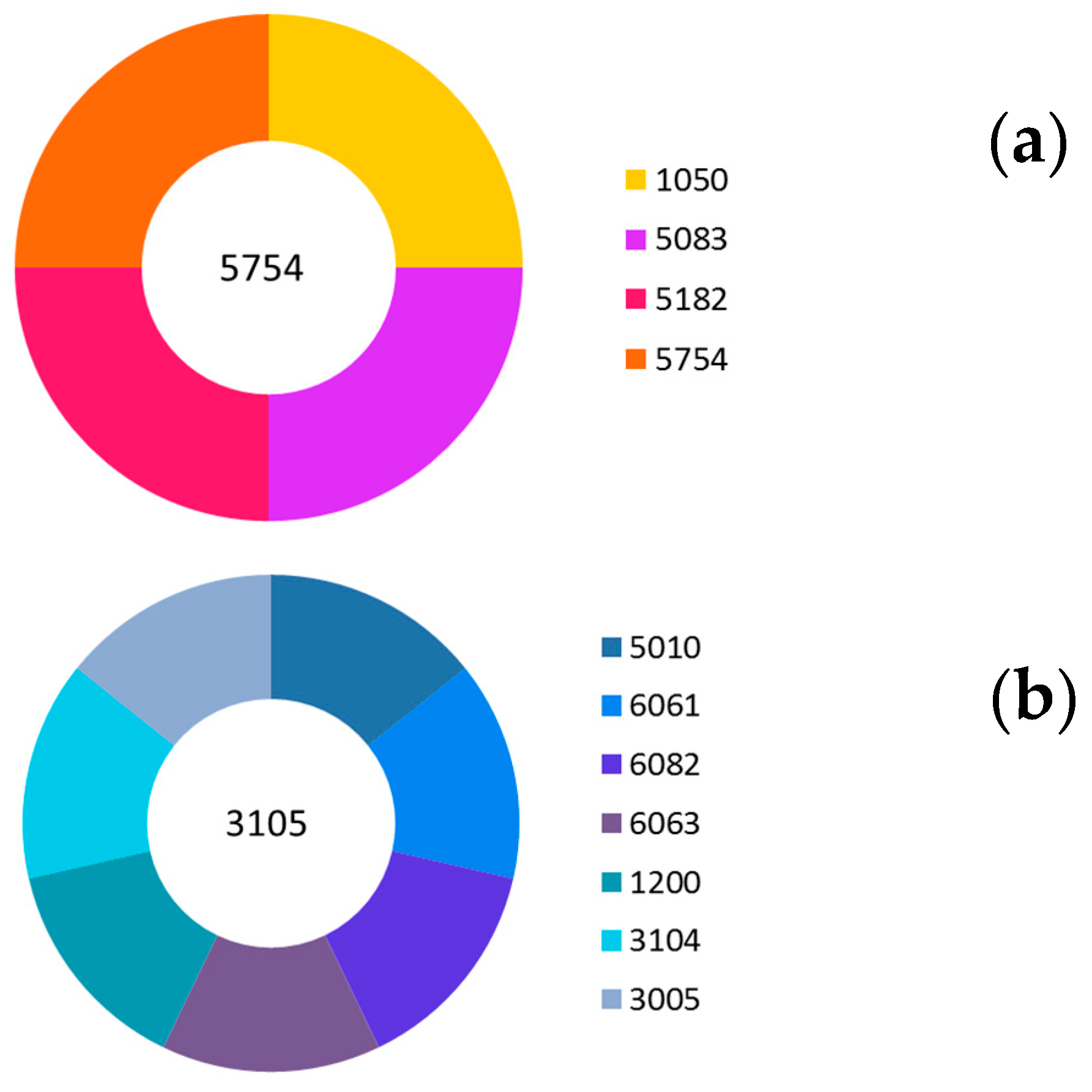

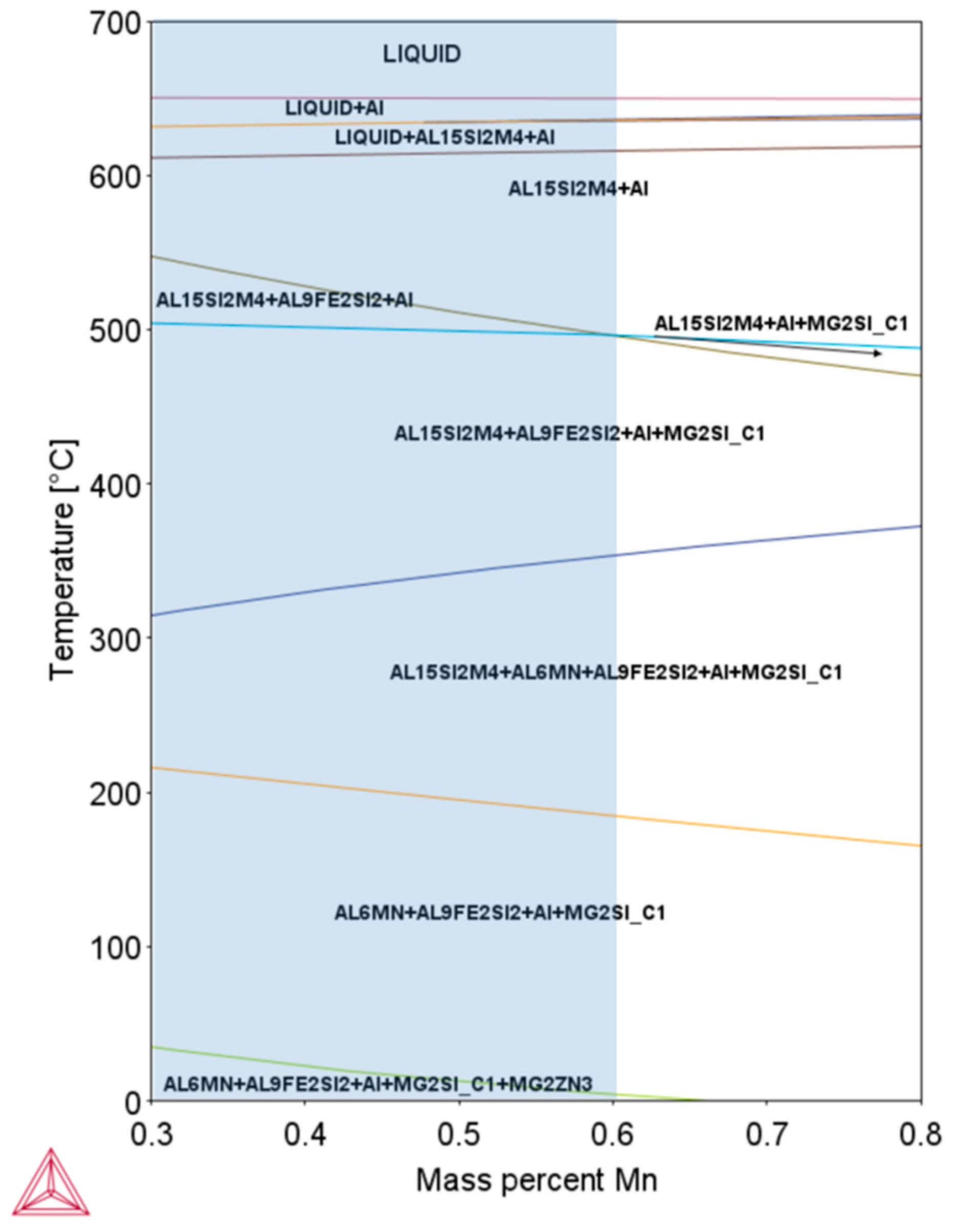
| Alloys | Si (%) | Fe (%) | Cu (%) | Mn (%) | Mg (%) | Zn (%) | Ti (%) |
|---|---|---|---|---|---|---|---|
| 1050 | 0–0.25 | 0–0.4 | 0–0.05 | 0–0.05 | 0–0.05 | 0–0.07 | 0–0.05 |
| 1200 | - | - | 0–0.05 | 0–0.05 | - | 0–0.1 | 0–0.05 |
| 3104 | 0–0.6 | 0–0.8 | 0.05–0.25 | 0.8–1.4 | 0.8–1.3 | 0–0.25 | 0–0.1 |
| 3005 | 0–0.6 | 0–0.7 | 0–0.3 | 1–1.5 | 0.2–0.6 | 0–0.25 | 0–0.1 |
| 3003 | 0–0.6 | 0–0.7 | 0.05–0.2 | 1–1.5 | - | 0–0.1 | - |
| 5083 | 0–0.4 | 0–0.4 | 0–0.1 | 0.4–1.0 | 4–4.9 | 0–0.25 | 0–0.15 |
| 5182 | 0–0.2 | 0–0.35 | 0–0.15 | 0.2–0.5 | 4–5 | 0–0.25 | 0–0.1 |
| 5754 | 0–0.4 | 0–0.4 | 0–0.1 | 0–0.5 | 2.6–3.6 | 0–0.2 | 0–0.15 |
| 5010 | 0–0.4 | 0–0.7 | 0–0.25 | 0.1–0.3 | 0.2–0.6 | 0–0.3 | 0–0.1 |
| 6061 | 0.4–0.8 | 0–0.7 | 0.15–0.4 | 0–0.15 | 0.8–1.2 | 0–0.25 | 0–0.15 |
| 6063 | 0.2–0.6 | 0–0.35 | 0–0.1 | 0–0.1 | 0.45–0.9 | 0–0.1 | 0–0.1 |
| 6082 | 0.7–1.3 | 0–0.5 | 0–0.1 | 0.4–1.0 | 0.6–1.2 | 0–0.2 | 0–0.1 |
| 5754 | 3105 | |||
|---|---|---|---|---|
| EN AW-5754 [8] | New | EN AW-3105 [8] | New | |
| Si (%) | 0–0.4 | 0–0.3 | 0–0.6 | 0.2–0.6 |
| Fe (%) | 0–0.4 | 0–0.4 | 0–0.7 | 0–0.5 |
| Cu (%) | 0–0.1 | 0–0.1 | 0–0.3 | 0–0.2 |
| Mn (%) | 0–0.5 | 0.2–0.5 | 0.3–0.8 | 0.3–0.6 |
| Mg (%) | 2.6–3.6 | 2.7–3.3 | 0.2–0.8 | 0.4–0.8 |
| Zn (%) | 0–0.2 | 0–0.2 | 0–0.4 | 0–0.2 |
| Ti (%) | 0–0.15 | 0–0.1 | 0–0.1 | 0–0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzouni, M.; Papaefthymiou, S. How to Design the Utilization of Larger Scrap Share in Aluminum Production. Mater. Proc. 2021, 5, 43. https://doi.org/10.3390/materproc2021005043
Bouzouni M, Papaefthymiou S. How to Design the Utilization of Larger Scrap Share in Aluminum Production. Materials Proceedings. 2021; 5(1):43. https://doi.org/10.3390/materproc2021005043
Chicago/Turabian StyleBouzouni, Marianthi, and Spyros Papaefthymiou. 2021. "How to Design the Utilization of Larger Scrap Share in Aluminum Production" Materials Proceedings 5, no. 1: 43. https://doi.org/10.3390/materproc2021005043
APA StyleBouzouni, M., & Papaefthymiou, S. (2021). How to Design the Utilization of Larger Scrap Share in Aluminum Production. Materials Proceedings, 5(1), 43. https://doi.org/10.3390/materproc2021005043







