1. Introduction
Aluminum (Al) is an essential technological material with excellent physicochemical properties and the ability to form light-weight alloys with many other elements, increasing in this way its strength and acquiring other useful properties [
1]. As a result of these properties, aluminum can be casted and/or thermomechanically treated to produce commercial products. The aforementioned parameters combined with data provided by the International Aluminum Institute [
2] justify the leading role of aluminum in almost every single aspect of human society [
3] and indicate the increase of its global demand in the forthcoming decades. Although numerous methods have been developed for the extraction of aluminum from its ores, its current commercial production method is based on two processes developed by the end of 19th century: the Bayer hydrochemical method, which produces pure alumina (Al
2O
3) from bauxite ores, and the Hall-Héroult electrolytic method, which produces pure aluminum metal using as a raw material metallurgical alumina [
4]. However, in recent years, the availability of good grade bauxite in Europe has diminished, and the price has correspondingly increased [
5]. This trend is also confirmed by the Bauxite inclusion in the EU’s critical raw materials list for 2020 [
6] making more evident the need for the utilization of alternative resources.
In the field of alternative Al sources, clays are reported as competitive candidates in Al production [
7]. Research on this field suggests the direct dissolution of these Al sources in strong inorganic acids [
8]. Based on the Aranda and Mastin technology [
9], AlSiCal project is a Horizon 2020 EU funded project that suggests a process for alumina and calcium carbonate production from aluminum rich resources with integrated CO
2 utilization, comprising: leaching of Al-rich resources in concentrated HCI, recovery of Al from the leach solution by crystallization of AlCl
3 6H
2O followed by calcination to produce alumina and to recover HCI.
Kaolin as a soft white clay with a high Al content, low-cost and wide availability, appears as an attractive candidate for this process. The utilization of kaolin in Al production has been the subject of research in the past [
10]. Kaolin is a 1:1 layer silicate consisting of Al octahedral and Si tetrahedral sheets that are linked together by strong ionocovalent bonds. These bonds do not enable the dissolution of the Al content at low retention times and temperatures that are industrially preferred and make the use of a pretreatment activation step essential for the settling of a novel and sustainable method for Al production. For the effective utilization of natural kaolin samples, the surface should be activated to ensure a high specific surface area and suitable chemical properties. The modification procedures that are mainly adopted for kaolin samples are mechanical activation, surface impregnation, thermal activation, chemical activation, and thermo-chemical activation [
11]. Modification via thermal activation, which leads to dehydroxylation, in clay minerals has attracted much interest [
7] due to the widespread use of these materials in a wide range of applications. Dehydroxylation of clays is achieved by the continuous loss of interlayer water and the subsequent discontinuous loss of structural water. This process results in a strong decrease in the basal distance and is associated with the collapse of the interlayer space and structural changes. Alumina extraction from thermal treated kaolin, metakaolin, has been widely investigated with a range of acid hydrometallurgical processes [
12,
13,
14]. Leaching of aluminum content from calcined kaolinitic clays with HCl or H
2SO
4 solutions followed by precipitation of aluminum salts and a thermal decomposition step that drives to the production of alumina has been an industrial applied process established in the last few [
15]. Although such process would provide high Al extraction, the high energy consumption and CO
2 footprint that is derived from the thermal treatment step are of great concern. In this study the feasibility of using untreated kaolin is examined. For this purpose, at first a comparison, on a metakaolin and a kaolin sample on the same leaching conditions, is presented, followed by leaching tests on untreated material.
2. Materials and Methods
2.1. Materials
In this study, two different samples of kaolin were examined, obtained from Kaolin und Quartzsandwerke GmbH and Co and IMERYS, previously named S&B, referred to as AMB and SB Kaolin. Both materials were dried, crushed and sieved to obtain particles smaller than 250 μm. A small proportion, derived through sampling, was dried at 100 °C for 24 h and was then chemically characterized via XRF. Mineralogical characterization was made by a X’Pert Pro diffractometer (PANalytical) with CuKa radiation.
2.2. Methods
Thermal Activation
Metakaolin production was conducted via a calcination step. Calcination was performed in a laboratory muffle furnace, under open air conditions. In each calcination test, a sample of SB kaolin was heated with a standard heating ratio of about 3 °C/min until the temperature of 850 °C was reached, before it was left at this temperature for 120 min and finally cooled down. The applied conditions were based on a previous work of NTUA [
13] on a sample of similar mineralogical characteristics.
2.3. Leaching
Experiments took place in an acid-resistant lab set-up including a 500 mL glass reaction vessel with a 5-neck lid, a laboratory heater equipped with a J-type thermocouple and the appropriate controller, and a glass condenser adapted to the vessel lid. A mechanical stirrer, with a PTFE shaft impeller, was used to keep the leaching slurry at a constant agitation speed. For the leaching agent, laboratory grade hydrochloric acid (>37%) and deionized water were used. When the acid solution in the reactor reached the predetermined temperature, the solid sample was inserted under constant stirring, signaling the start of the experiment. In kinetic experiments, samples were taken at defined time intervals while at the end of experiment solid/liquid separation via vacuum filtration was performed. The final pregnant liquid solution (PLS) and the intermediate samples were analyzed by AAS with the use of Perkin Elmer 2100 Atomic Absorption Spectrophotometer, while the residue was dried.
In this work, the effect of thermal treatment, retention time (0–96 h) and leaching media concentration (1, 5.89 M) on the extraction of aluminum was studied during the leaching of two kaolin samples with an HCl solution under a constant stirring rate of 300 rpm. In addition, the co-dissolution of Si was monitored in all experiments.
3. Results and Discussion
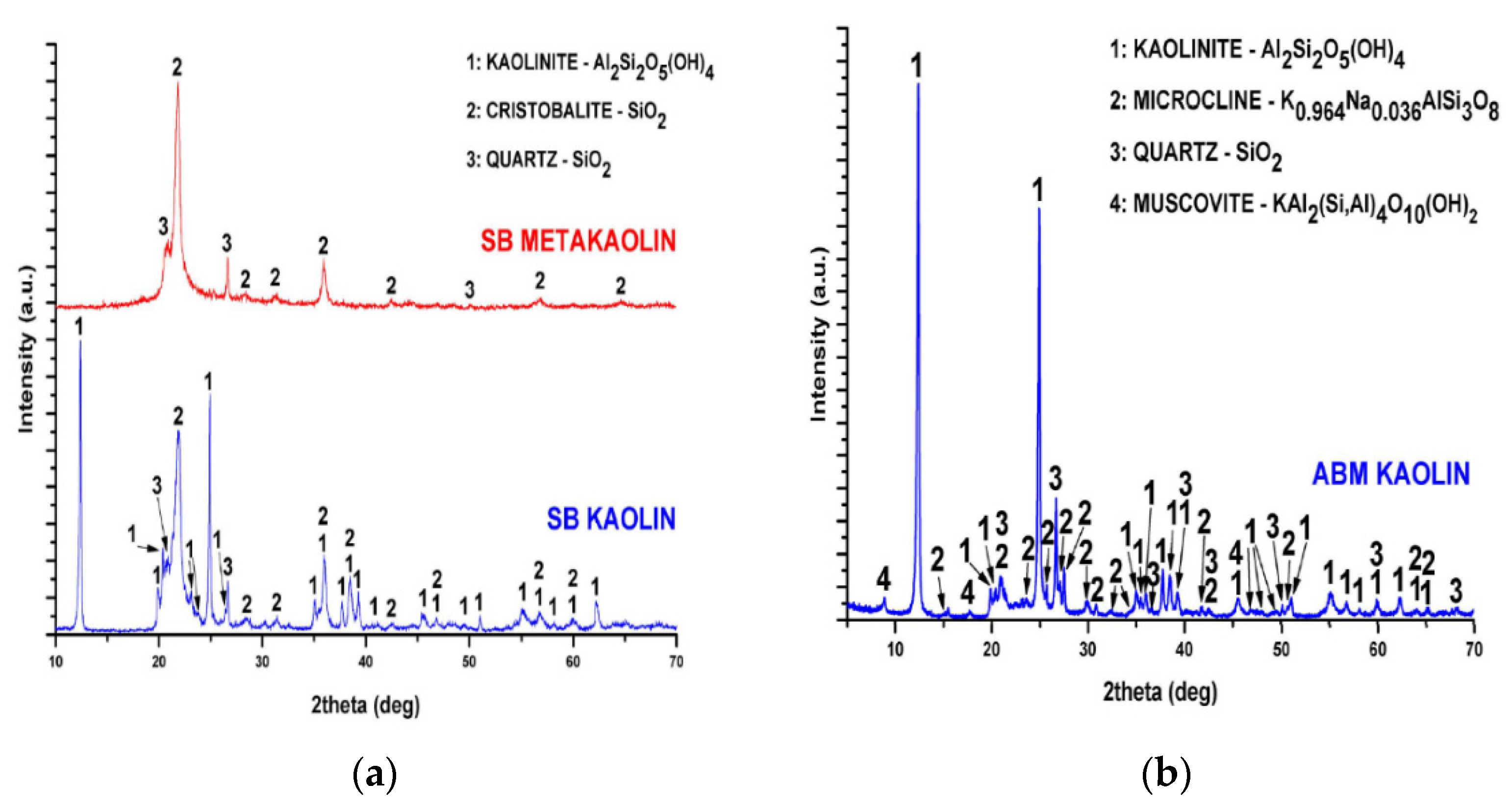
The chemical composition (on a dry weight basis) of AMB kaolin and SB kaolin and SB metakaolin are shown in
Table 1 and the X-ray diffractogram in
Figure 1a,b. According to
Table 1 AMB kaolin presents a higher Al content in comparison with SB kaolin, while K and Na oxides where traced. Mineralogical characterization suggests that these alkaline oxides are included in the form of microcline and muscovite, two aluminate phases that contribute to the total Al content of AMB Kaolin along with kaolinite. The high Al content of AMB Kaolin make it an attractive material for Al production. On the other hand, SB kaolin includes Al only in the form of kaolinite and Si in the form of cristobalite and quartz. Due to its simpler structure, SB Kaolin was used for the thermal treatment process and the production of metakaolin that is described in the following session. From the comparison of the SB kaolin and Metakaolin spectra it is observed that kaolinite peaks that were detected in SB kaolin are absent in SB Metakaolin, certifying the transformation of kaolinite to an amorphous phase that cannot be detected in the XRD analysis. Si phases, cristobalite and quartz are not affected by the calcination and are detectable in the SB metakaolin spectra.
3.1. Effect of Low HCl Molarity on Untreated and Thermal Treated Kaolin
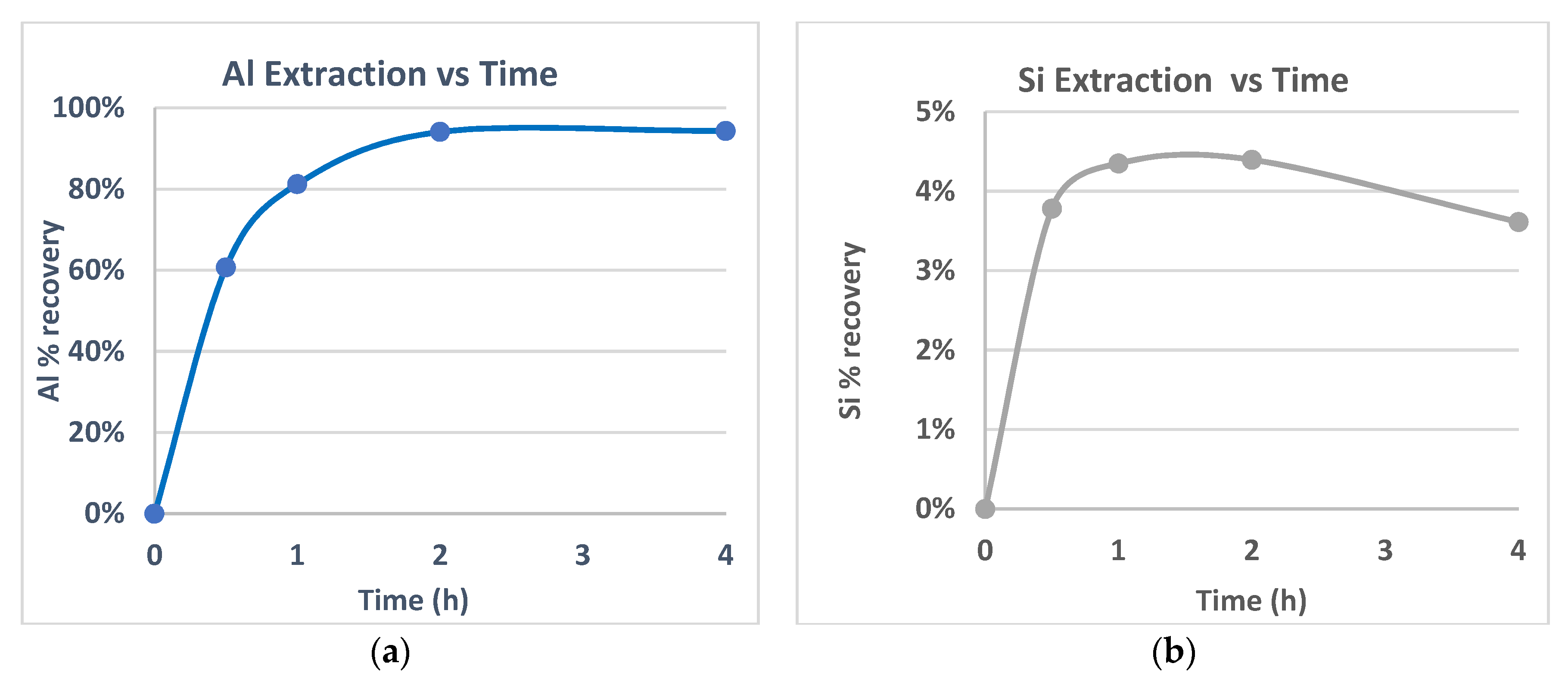
The wt% extraction of Al and Si as a function of time are illustrated in
Figure 2a,b for SB Metakaolin and in
Figure 3a,b for SB Kaolin Al and Si% wt extracted values.
As was expected from bibliographic data [
12], SB metakaolin follows a fast dissolution mechanism, even with the use of a low molarity HCl leaching agent, achieving a 94 wt% Al extraction that is kept constant after that time. Si co-dissolution is low, with its highest value, near 5 wt%, noted at 2 h and decreased at a longer retention time due to its re-precipitation. In the case of untreated SB Kaolin, as demonstrated in
Figure 3, Al dissolution rate was very low, leading at 4 h of treatment to an Al recovery under 4%. While Si wt% extraction is also, as depicted for SB Metakaolin, near 5% at a retention time of 4 h. Comparing the Al extraction diagrams of untreated and thermal treated material, in the same applied condition, the advantage and the importance of the calcination step is confirmed. Nevertheless, the disadvantages and challenges related to a calcination process, described extensively in the introduction section, warrant the need to further study the leachability of untreated kaolin.
3.2. Effect of Periodical Intensification on Untreated Kaolin
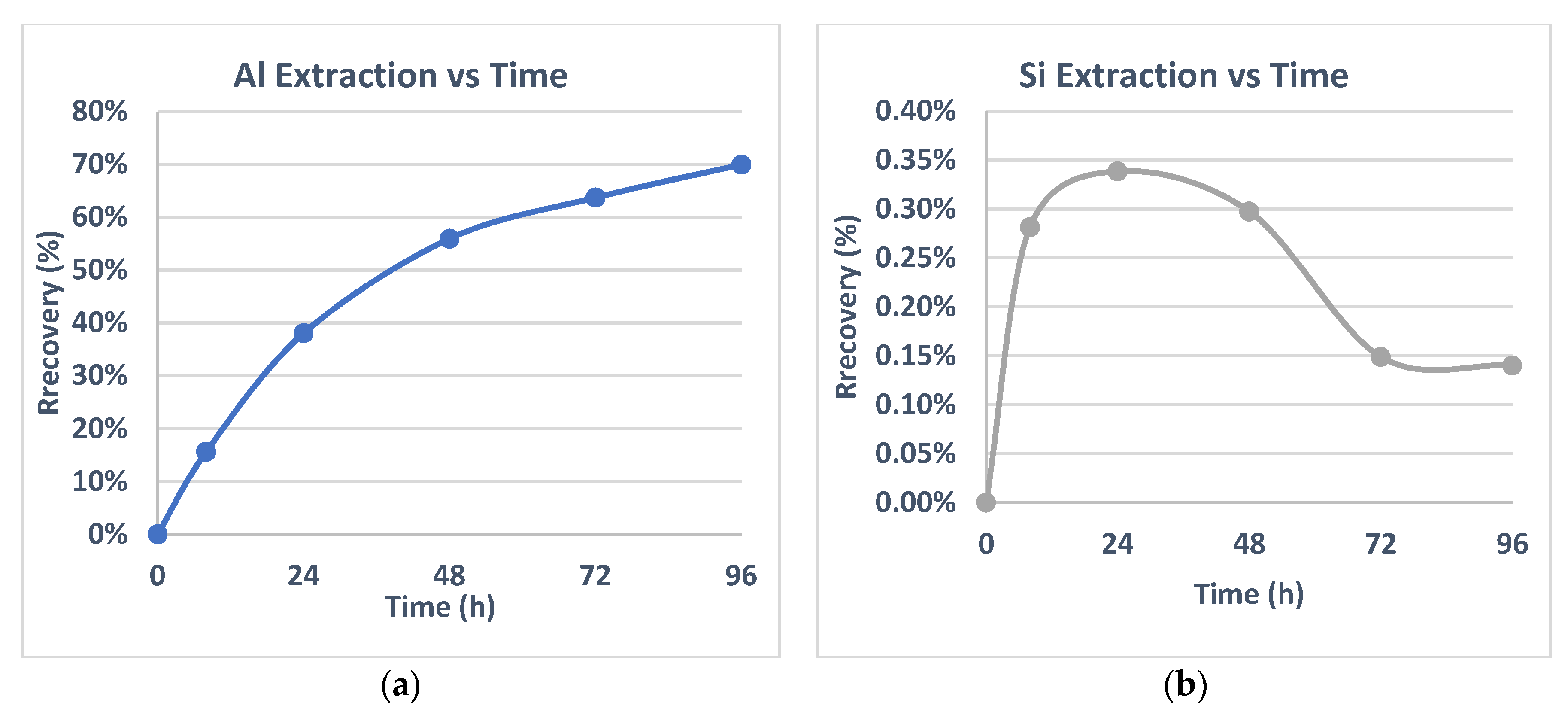
As a first step the leachability of untreated material on a longer retention time of 96 h, at a 1 M HCl concentration, was tested in AMB kaolin, with the wt% recovery as a function of time demonstrated in
Figure 4a for Al and
Figure 4b for Si.
A long-term treatment time seems to act as a crucial factor in Al extraction. More specifically, Al% extraction is enhanced every 24 h with a 8 wt% addition on extraction value, leading at 96 h to a final extraction near 35%, while Si dissolution is ranged in very low extraction rates. In this leaching series the importance of periodical intensification on kaolin dissolution, by the application of a longer treatment time, is noted. Huertas [
16] presented Al extraction diagrams that followed the same trend as the one illustrated in
Figure 4a. In his study on the dissolution kinetic model, it is mentioned that kaolinite overall dissolution rate is the sum of the rates of a short-term dissolution reaction, near 100 h, and of a linear dissolution process. The high kaolinite dissolution rate relates to different processes, such as dissolution of fine-grained materials or highly strained areas and defects on large grains. These processes that are linked to a high dissolution rate take place at short retention time and from that moment, the dissolution proceeds at a constant rate.
3.3. Effect of Chemical Intensification on Untreated Kaolin
In
Figure 5a the extraction of Al as a function of time with the use of the azeotropic mixture of HCl is provided. Comparing the Al extraction in
Figure 4a and
Figure 5a, the impact of chemical intensification on Al dissolution is noticed.
The application of the azeotropic mixture on untreated kaolin for a retention time of 96 h led to an Al extraction of 70%. The use of a higher HCl concentration acts as a catalyst and reduces the needed retention time. It is worth mentioning that the Al extraction, achieved in the 1 M test at 96 h, is achieved at 24 h of leaching with the azeotropic HCl. Si co-dissolution is ranged in very low extraction rates, near 0.15%.
Under acidic conditions, the proton promoted mechanism controls dissolution rate by the breaking of Al–O bonds, through the formation of AlOH
2+ groups [
16]. Dissolution rate, for many minerals such as kaolin, within certain pH ranges, is proportional to a fractional power of the hydrogen ion activity. This suggests that the use of a higher HCl concentration, and consequently a higher hydrogen ion concentration, is connected with the increase in the dissolution rate, which is also noted in this study [
17]. Beyond retention time and leaching agent concentration, temperature is a parameter that according to Cama [
17] has a significant impact on the Al dissolution rate, and more particularly is empowered by their value increase. A leaching series on the effect of thermal intensification is proposed as a field of future research.
4. Conclusions
Data provided by this study and previous works, conducted on thermal treated kaolin leachability, confirm that metakaolin can be a competitive candidate in alumina production via the acidic route. The abundancy of kaolin deposits, in combination with the mild conditions, needed in the leaching step for the total dissolution of aluminum content, are considered as two very attractive advantages of the applied method. However, the calcination step, preceding the leaching step, is a major drawback, connected with high energy consumption and CO2 footprint. The tests conducted on untreated material led to an intermediate Al recovery that could possibly lead to a higher concentration in Al pregnant liquid solution with the application of a higher pulp density. However, the use of a high HCl concentration and a retention time of nearly 100 h could not be characterized as a profitable method and needs optimization. In this path, an application of a higher temperature with a shorter retention time could provide more attractive results that would lead to an industrial applied method.