1. Introduction
Antimony is a metal with strategic importance and a wide range of applications. The research about antimony extraction is significant due to the fact that it is rarely found in nature alone, but mostly in association with other metals [
1]. Antimony compounds are used in almost all modern industries and its future uses relate mainly to the developing technological field. It has been included in the EU critical raw materials list, considering its economic and strategic importance and high supply risk, given that worldwide, 75% of the total antimony is produced in China [
2].
There are several antimony deposits in Greece; however, none are currently exploited. When beneficiating mixed sulphide ores, such as that of Kassandra (Olympias and Stratoni) mines, Chalkidiki, NE Greece, antimony is collected together with lead in galena concentrate. Although antimony is a critical element, it is considered as an impurity and, as in the case of arsenic, penalties are applied by customers when their total content in the concentrate exceeds certain values.
For the extraction of antimony in this study, leaching with strongly alkaline sodium sulphide solution is examined following a process similar to that applied at the former Sunshine Mine Company for the treatment of silver containing copper, antimony and sulphide concentrates [
3]. Despite the significant differences of Olympias galena concentrate and the Sunshine Mine argentiferous copper concentrate, there were some chemical similarities rendering the application of the method still feasible for galena concentrates [
4].
During sulphide leaching, sodium sulphide dissociates, resulting in the formation of S
2−, HS
− and H
2S
(g) with their relative presence dependent on solution pH. Sulphide ions dominate when pH is greater than 10. The alkaline environment is necessary to prevent sulphide ion hydrolysis and the release of toxic H
2S gas [
5]. Antimony minerals existing in galena concentrates as either antimony sulphide (Sb
2S
3, stibnite) or mixed sulphides such as bulangerite (Pb
5Sb
4S
11) are dissolved in alkaline sodium sulphide solutions to produce various thioanions such as thioantimonite (SbS
33−) or thioantimonate (SbS
43−), depending on reaction conditions. The main reaction describing bulangerite dissolution is:
When there are an insufficient number of sulphide ions in the solution, NaOH will dissolve antimony according to the equation.
2. Materials and Methods
A representative sample of galena concentrate was obtained from Olympias Mine located in Chalkidiki (NE Greece). The grain size distribution was determined by the dry sieving of coarse fractions and laser particle analysis (Malvern microplus laser particle size analyser, Malvern Panalytical Ltd., Malvern, UK) of finer fractions. A subsample of 100 g was finely ground and subjected to chemical and mineralogical analyses. Chemical analysis was performed by digestion followed by analysis of the solution by atomic absorption spectroscopy (AAS, PinAAcle 900T, PerkinElmer, Akron, OH, USA) and ICP-OES (Optima 7000, PerkinElmer, Akron, OH, USA), whereas the X-ray fluorescence technique (XRF, SPECTRO-XEPOS, SPECTRO, Kleve, Germany) was applied for the measurement of trace elements. Mineralogical analysis was performed by X-ray diffraction analysis (XRD, D8 Focus, Bruker, Billerica, MA, USA) and scanning electron microscopy (SEM) using a Jeol 6380LV (JEOL Ltd., Tendo-shi, Japan).
The galena concentrate was then subjected to alkaline sodium sulphide leaching, in order to extract antimony and arsenic. All experiments were conducted in a 500-mL five-necked, round-bottomed glass split reactor, which was fitted with a glass stirrer, a vapour condenser and a thermometer. In all of the experiments, a constant stirring speed was applied to ensure suspension of the particles. Heating was provided by a heating mantle and the temperature of the solution was controlled by a Pt-100 sensor. Leaching experiments were performed using sodium sulphide (100, 150 and 200 g/L) and sodium hydroxide (30 or 50 g/L) solution, at 5, 10 and 15% pulp density and temperatures of 90, 98 and 104 °C. Each run lasted 240 min and samples were taken at certain times as well as at the end of the experiment for chemical analysis of Sb and As. The solid residues were filtered under vacuum and analyzed with XRD. All leach solutions were analyzed by AAS and ICP-OES.
3. Results and Discussion
3.1. Sample Characterisation
Galena concentrate is a very fine material with d
90 value equal to 190 μm, d
10 = 2 μm and d
50 = 41.9 μm. The results of chemical analysis are given in
Table 1.
As seen in
Table 1, the Olympias galena concentrate mainly consists of Pb (52.89%), S (17.39%), Sb (9.91%), Fe (4.47%), As (2.79%) and Zn (1.73%).
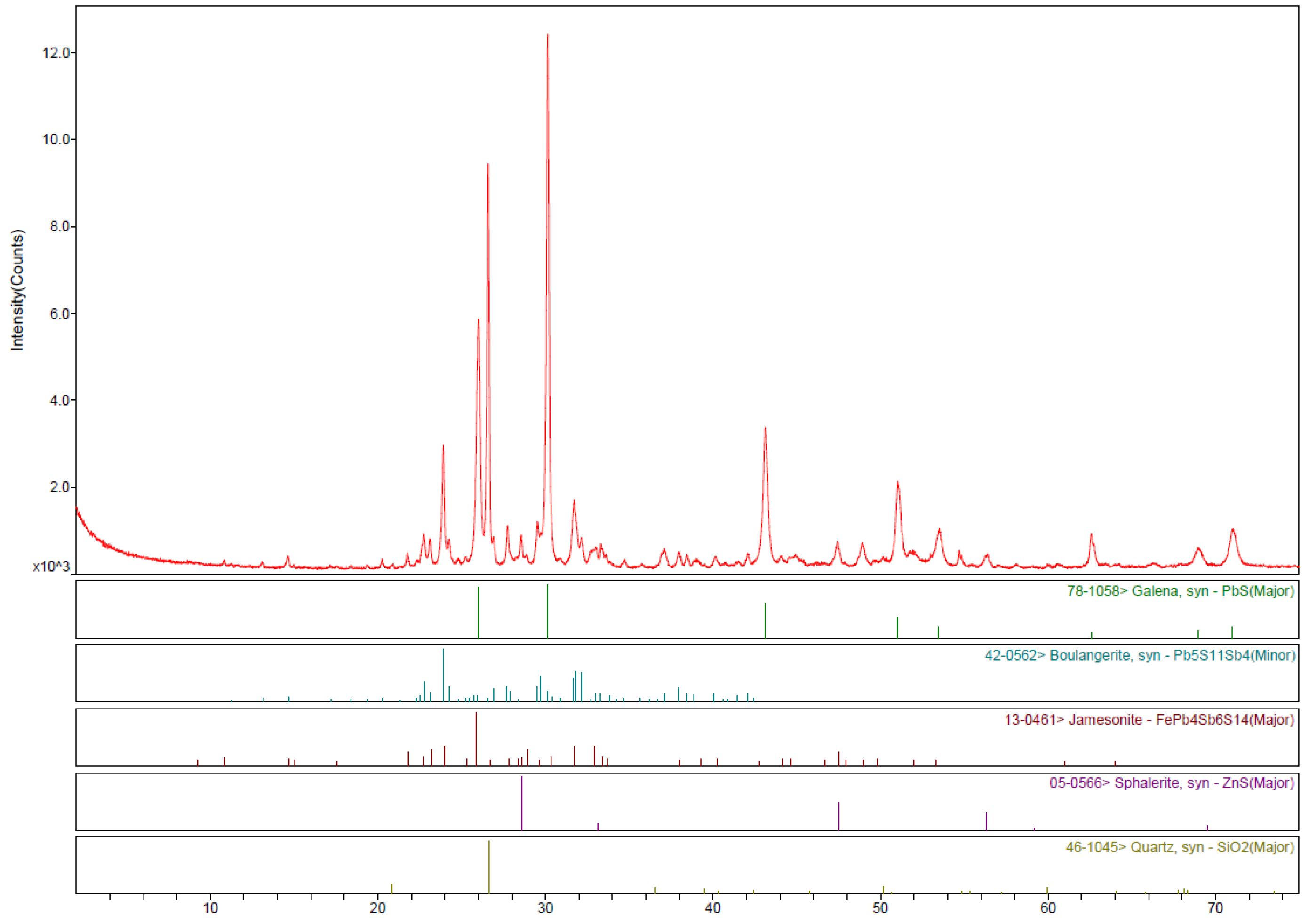
The main phases identified by XRD analysis are galena (PbS), boulangerite (Pb
5Sb
4S
11), jamesonite (FePb
4Sb
6S
14), sphalerite (ZnS) and quartz (SiO
2) (
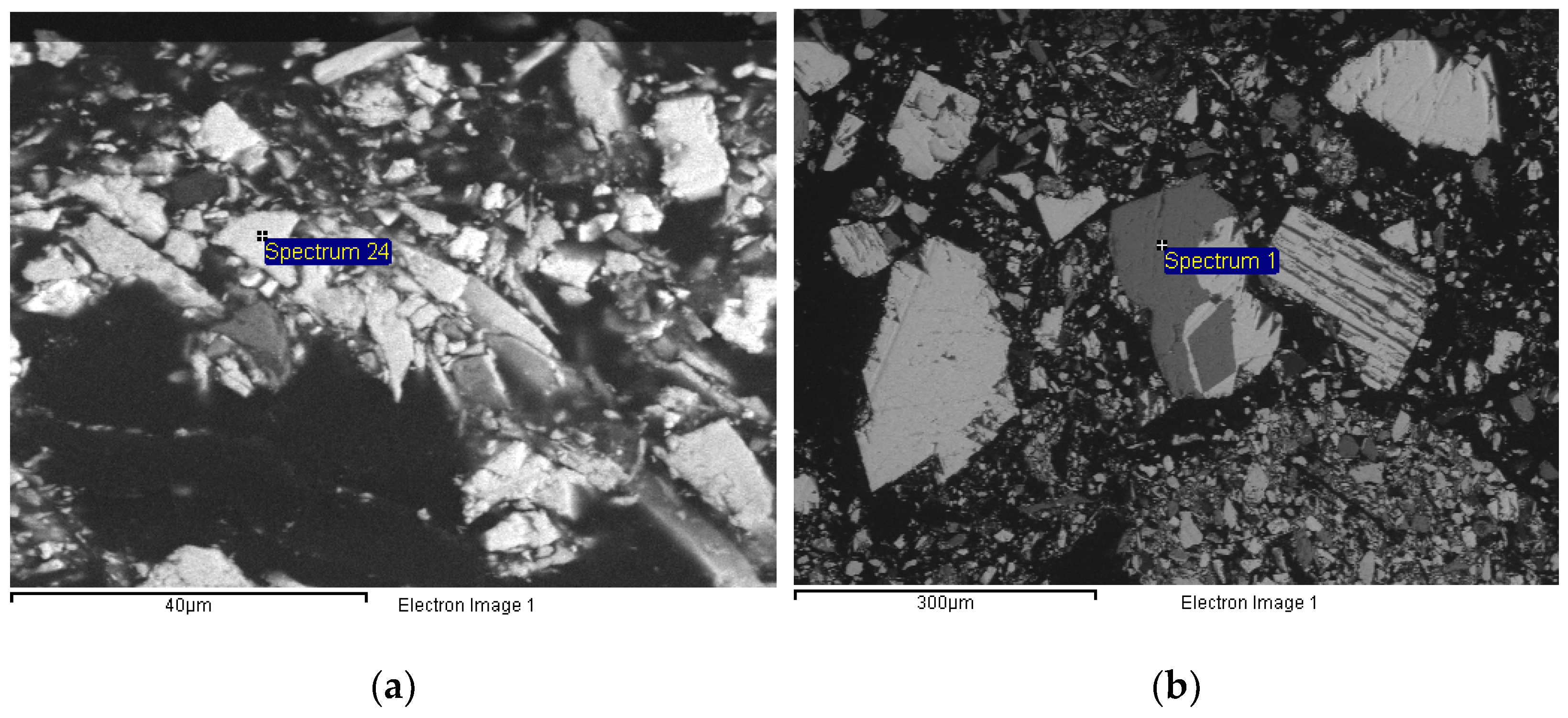
Figure 1). Furthermore, SEM/microprobe analysis confirmed the presence of arsenopyrite (FeAsS), pyrite (FeS
2), and burnonite (CuPbSbS
3) (
Figure 2).
3.2. Leaching
The results of Sb and As extraction at 4 h leaching time for all of the experiments performed are given in
Table 2. The kinetics of Sb and As extraction from galena concentrate vs. Na
2S concentration at 50 g/L NaOH concentration, a temperature 98 of °C and an S/L ratio of 0.1 kg/L are given in
Figure 3a,b, respectively.
As seen in
Table 1, the increase in Na
2S concentration in the leaching solution and temperature results in the increase in Sb extraction. Meanwhile, an increase in the S/L ratio results in the decrease in Sb extraction. Maximum Sb extraction (99.09%) was accomplished at 250 g/L Na
2S and 50 g/L NaOH concentrations in the leaching solution, a temperature of 98 °C and an S/L ratio of 0.1 kg/L. Under the experimental conditions applied, the kinetics of Sb dissolution are fast when the Na
2S concentration is high, reaching values close to the respective maximum values at around 1 hour (
Figure 3a). The Sb extraction kinetics at the lowest Na
2S concentration investigated (100 g/L) is slower. Concerning As extraction, it is always very low, ranging from 2.15 to 4.05%. This is attributed to the presence of As in galena as arsenopyrite, which is practically insoluble in the alkaline sodium sulphide solutions used in the present study.
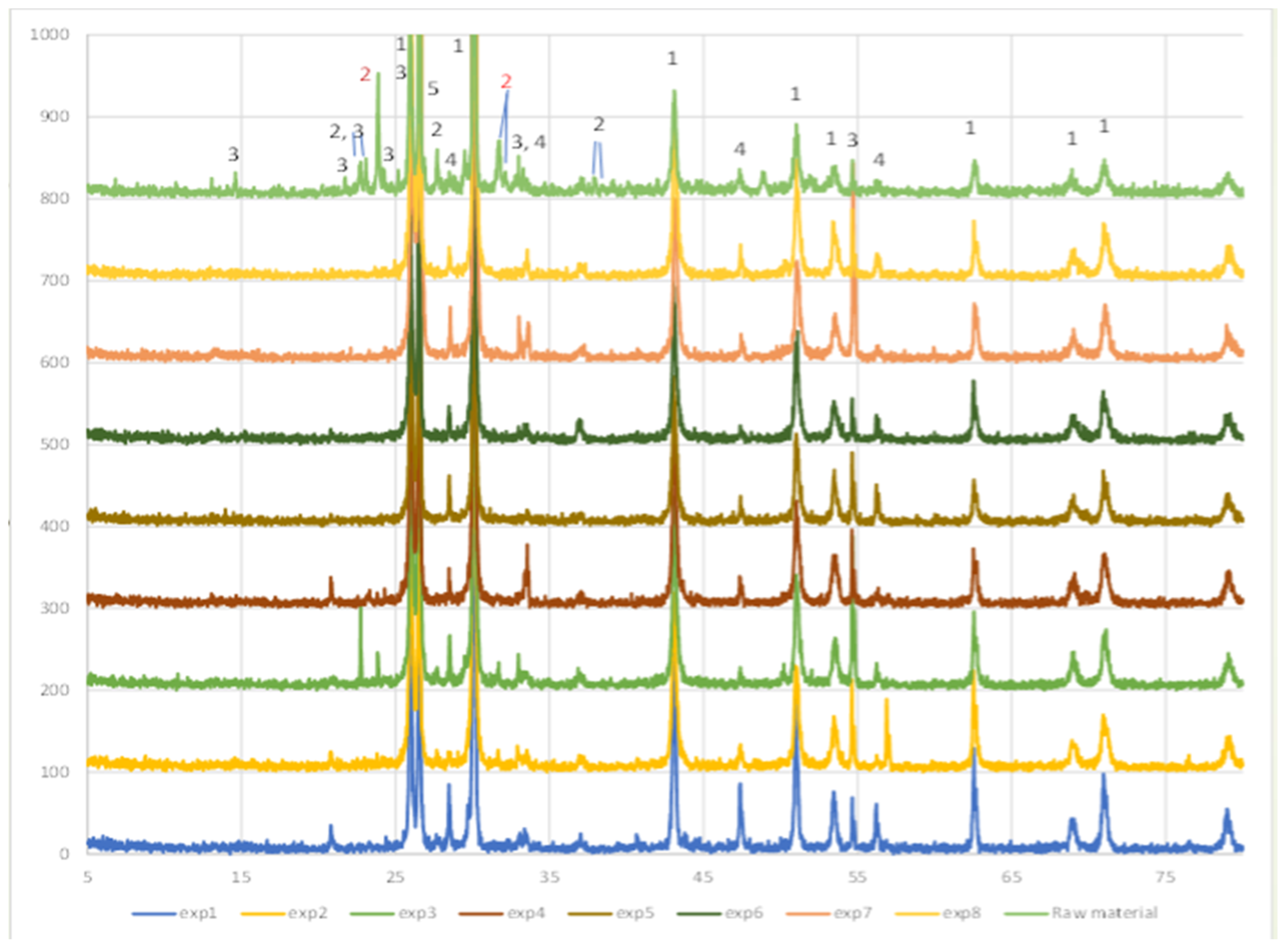
Solid leach residues have been further subjected to X-ray analysis with the respective patterns given in
Figure 4 in comparison with the initial galena concentrate pattern. As seen in
Figure 4, the peaks of boulangerite (Pb
5Sb
4S
11) disappeared as the antimony was dissolved in almost all of the tests that have not been performed under the mildest extraction conditions (temperature of 90 °C and Na
2S concentration of 100 g/L).
4. Conclusions
The extraction of antimony from Sb-bearing galena concentrates is of great importance in order to avoid the penalties applied in the market, as it is considered an impurity, and also due to its market value as it is a critical raw material. The Olympias galena concentrate sample used in this study contains 9.91% of Sb and 2.79% of As mainly in the form of boulangerite (Pb5Sb4S11) or jamesonite (FePb4Sb6S14) and arsenopyrite (FeAsS), respectively. The aim of this work was to find the optimal conditions to achieve maximum extraction of both Sb and As.
Based on the experimental results, it has been indicated that almost 100% antimony extraction can be achieved by leaching with sodium sulphide in alkaline conditions. Maximum Sb extraction (99.09%) is accomplished at 250 g/L Na2S and 50 g/L NaOH concentrations, a temperature of 98 °C and a S/L ratio of 0.1 kg/L. Arsenic extraction is always very low, ranging from 2.15 to 4.05%, which is attributed to the presence of As in galena in the form of arsenopyrite, which is practically insoluble in alkaline sodium sulphide solutions. XRD analysis of solid residues confirmed the absence of Sb-bearing phases.
Author Contributions
Conceptualization, A.X. and D.D.; methodology, A.X. and A.K.; validation, A.K., R.A.M. and P.O.; experimentation, R.A.M., A.K., P.O. and E.T.; data curation, R.A.M.; writing—original draft preparation, R.A.M.; writing—review and editing, A.K. and A.X.; visualization, R.A.M.; supervision, A.X.; project administration, A.X.; funding acquisition, A.K. and A.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hellas Gold S.A.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge Hellas Gold SA for funding this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lide, D.R. CRC Handbook of Chemistry and Physics, 87th ed.; Taylor & Francis: Abingdon-on-Thames, UK, 2007. [Google Scholar]
- Anderson, C.G. Antimony Production and Commodities. 2018. Available online: www.researchgate.net/profile/Corby-Anderson/publication/299133344_Antimony_Production_and_Commodites/links/5a7e1dcbaca272a73765cb27/Antimony-Production-and-Commodites.pdf (accessed on 5 March 2022).
- Anderson, C.G. The metallurgy of antimony. Geochemistry 2012, 72, 3–8. [Google Scholar] [CrossRef]
- Anderson, C.G. Hydrometallurgy at the Sunshine Mine Metallurgical Complex. 1993. Available online: www.researchgate.net/publication/287997283_Hydrometallurgy_at_the_Sunshine_Mine_Metallurgical_Complex (accessed on 5 March 2022).
- Samuel, A.A. Antimony Recovery from Complex Copper Concentrates through Hydro- and Electrometallurgical Processes. 2013. Available online: www.diva-portal.org/smash/record.jsf?pid=diva2%3A990544&dswid=5952 (accessed on 5 March 2022).
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).