Abstract
Primary world nickel production in 2020 was 2430.7 kt Ni; 69% (1677.7 kt) of them came from oxidized nickel ores (laterites) and 31% from sulfides. Production-wise, 87.7% of the 1677.7 kt came from pyrometallurgical and 12.3% from hydrometallurgical processes. For a long time, Fe-Ni had a 20–40% Ni analysis, but in 2006 a new Fe-Ni quality came into the scene. This is the nickel pig iron (NPI) with 2.5–5.5% Ni that comes from laterite smelting in the blast furnace (B/F). Eventually, the advantages of the R/K-E/F process led to its dispersion in China and Indonesia and resulted in an NPI production with 3–12% Ni. The NPI production in these two countries climbed from zero in 2000, to 1060 kt Ni in 2020 and also stainless-steel production from 5.5% to 67.2%, respectively, of the world’s SS production. The integration of Ni industry with SS production, the economy of scale, the low labor cost, the high Ni content of Indonesian laterite, and the loose environmental laws, reduced significantly the NPI production cost. The addition of SS and/or electric energy production units for cost reduction has been adapted from other Fe-Ni producers, as well. Hellenic Minerals in Cyprus after two years of successful industrial tests is in the commissioning state of a Heap Leaching-Solvent Extraction-Crystallizer (HL-SX-CR) unit for NiSO4.6H2O production. The high demand for Ni and NiSO4.6H2O in the last few years has changed the prospects of Ni laterite hydrometallurgical processing. Regarding the R/K-E/F process used in Greek Fe-Ni, it is characterized by its worldwide acceptance and reliability (more than 77% of world Fe-Ni production comes from this process). Other advantages are the use of all types of laterites and fuels, it has a high metallurgical recovery, and the plant has its own port. However, it is a high energy-consuming process, and it does not recover Co. The Greek laterite, in particular, has the lowest Ni% among global Fe-Ni producers and because of this, electric energy (MWh/t Ni) and wages (wages/t Ni) per ton of Ni are high, making Larco’s viability difficult. The only way to overcome the issue with specific electricity consumption is to enrich the local ores by blending them with imported high grade Ni ores. Other negatives were the excessive electric energy price it had to pay to a dominant energy supplier and the very frequent changes of its administration.
1. Introduction
In this work, the metallurgical treatment methods of nickeliferous laterites are examined and among them the Greek ferronickel (Fe-Ni) production process. Nickel laterites belong to one of the two big nickel ore categories. The other category is the sulfides. As it is known, 72.2% of Ni on earth surface resources is found in laterites and the rest 27.8% in sulfides [1,2]. Although up to 2004 only 42% of world Ni production was coming from laterites [1], in 2020 it reached 69%. As shown in Table 1 average quality of laterite resources is 1.28% Ni and of sulfides 0.58% Ni. The countries with 69% of laterite resources are New Caledonia (23%), the Philippines (17%), Indonesia (16%), and Australia (13%). Laterites with Ni% ≥ 0.9%, and sulfides with Ni% ≥ 0.3% are considered to be economic [1].

Table 1.
World Ni resources, (laterites and sulfides) [1].
According to their formation, Ni laterites are of three types: Lemonitic (Fe,Ni)O.OH.nH2O, garnieritic (Mg,Ni)SiO3.nH2O and intermediate type (serpentinic) (Mg,Fe,Ni)6Si4O10(OH)8. Limonitic type, have around 0.9–1.5% Ni and they are rich in Fe and poor in Mg and Si. Garnieritic type have Ni% ≥ 2.0%, and they are rich in Mg and Si and poor in Fe [3]. This classification is based on their Fe and MgO chemical analysis, as follows:
| Type A: Garnierites | (Fe < 12%, MgO > 25%) |
| Type B: Lemonites | (Fe > 25%, MgO < 10%) |
| B1: “ | (Fe > 32%, MgO < 10%) |
| B2: “ | (25% < Fe < 32%, MgO < 10%) |
| Type C: Intermediate | (12% < Fe < 25%, 10% < MgO < 35%) |
| C1 “ | (12% < Fe < 25%, 25% < MgO < 35%) |
| C2 “ | (12% < Fe < 25%, 10% < MgO < 25%) |
Another way of classification is according to their geological profile, from the dry type (as those of Australia) to those of wet tropical areas (as those of Indonesia), (Table 2) [1].

Table 2.
Laterite classification, according to its geological structure, and chemical analysis [1].
Due to their formation, (a heterogeneous mixture of iron hydroxides and magnesium hydro-silicates), laterites cannot usually be beneficiated by physical methods. So, the only way of metal separation from the gangue is the metallurgical methods.
Nickel analysis of laterites used in Fe-Ni production is given in Table 3 and Greek laterites are the lowest in Ni% of all Fe-Ni producers, except Myanmar ore which was not available. As shown in Table 3, Ni% analysis of Greek laterites is about 0.95% Ni, whereas the mean Ni% value of the rest Fe-Ni producers in the table is 2.02% Ni, i.e., more than 100% higher than that of Larco. This means that the specific electric energy consumption of Larco, (MWh/t Ni), is more than double of that of competition and the productivity of Larco (wages/t Ni) is less than half of the competition. This is a big disadvantage of Greek Fe-Ni, making Larco’s viability difficult unless it can find a better-quality ore.

Table 3.
Ore Ni% analysis of Fe-Ni producers [1,2,4,5,6,7,8].
Regarding exploitation of Pacific Ocean polymetallic nodules and seabed mining close to Norway, there were recently some announcements, but there is no information about environmental and technical-economical aspects of these projects, and also there are voices calling for a moratorium on deep-sea mining [9]. So, we cannot say more about it.
2. Metallurgical Methods
Nickel extraction from laterites is achieved by pyro- and hydro-metallurgical methods.
With the pyrometallurgical methods Ni is produced:
- In the form of a ferronickel alloy, (Fe-Ni), with 20–40% Ni, by the rotary kiln-electric furnace (R/K-E/F) method.
- In the form of a nickel pig iron, (NPI), with 3–12% Ni, during the last 15 years, by the R/K-E/F, the B/F, and the E/F methods.
- In the form of Ni matte (Ni3S2), by the R/K-E/F and the B/F method.
With the hydrometallurgical methods is produced:
- In the form of metallic Ni, NiSO4.6H2O or NiO, by H2SO4 leaching in autoclaves under high temperature and pressure (High Pressure Acid Leaching (HPAL)).
- In the form of metallic Ni, NiSO4.6H2O or NiO, by atmospheric leaching (AL) with H2SO4 and up to 100 °C and in heaps (HL) with H2SO4 leaching and solvent extraction (S/X).
- In the form of NiO, by a combination of a pyro- and a hydrometallurgical process, i.e., ore roasting reduction and then ammonia leaching of calcine under atmospheric pressure (Caron Process).
3. Nickel Production by Pyrometallurgical Processing
3.1. Ferronickel Production with 20–40% Ni
Ferronickel (Fe-Ni) production from oxidized Ni ores by using reduction methods is achieved according to thermodynamics [10]. So, in roasting reduction of the ore at 850–1000 °C, we have a simultaneous reduction of Ni, Co, and Fe oxides. During the next step of smelting, a Ni-Fe-Co alloy and slag are formed and not only Ni and slag [10] (p. 431). In addition, if the reducing agent C is in excess in the metallurgical mixture or if we have intensive smelting reduction, as it happens in the B/F, then C and Si will pass into the alloy. World primary Ni production in 2020 is given in Table 4, by country, kind of ore, company and treatment method, for laterites. As shown in the table it was 2430.7 kt Ni, and from them, 1677.7 kt or 69%, i.e., more than two-thirds are coming from laterites, and the rest 753 kt or 31% from sulfides. In addition, the Ni production from laterites, was 87.7% or 1471 kt by pyro- and 12.3% or 206.7 kt by hydrometallurgical processes. From the pyro- processes the R/K-E/F method is leading with the production of Fe-Ni and from the hydrometallurgical ones, the only method operating in 2020 was high-pressure acid leaching (HPAL), producing Ni, Co, NiSO4.6H2O, and NiO. In addition, from the 1471 kt Ni of pyrometallurgical processes, only 33.3 kt Ni was Ni matte and the rest either Fe-Ni with 20–40% Ni or NPI, i.e., Fe-Ni with 3–12% Ni. With the R/K-E/F method, Fe-Ni with 20–40% Ni is usually produced by partial reduction in the R/K and then smelting reduction in the E/F or complete reduction in the R/K and melting in the E/F. Smelting reduction is also applied in B/Fs and E/Fs in China and Indonesia.

Table 4.
World primary Ni production by country, company, ore category, and processing method of laterites (2020).
The R/K-E/F method is applied for Fe-Ni with 20–40% Ni in Dominican Republic, Indonesia, Brazil, Columbia, North Macedonia, Kosovo, New Caledonia, Greece, Japan, Ukraine, South Korea, Guatemala, and Myanmar. The Anglo-American plant of Loma de Niquel in Venezuela has been out of operation since 2015 [11]. Baro Alto plant of Anglo American in Brazil started in 2011 and during the last 5 years (2016–2020) it had 35 kt Ni/y production, whereas the Vale Onca Puma plant in Brazil started in 2010 and its production during the last 5 years was 11.6–24.7 kt Ni/y. At Euronickel in North Macedonia, the ore is pre-dried, crushed, ground, pelletized and fed on a grate (Lepol grate), for drying and pre-heating to 800 °C from the R/K gases [12].
In Dominican Republic, Falcondo is achieving complete reduction in a shaft furnace, (S/F), melting in the E/F producing FeNi with 35% nickel [13]. In New Caledonia, Koniambo is using a fluosolids partial reducer, (F/R), and the calcine is going to the E/F for smelting reduction producing FeNi with 35% nickel. The feed ore is 2.5% Ni with a cutoff grade of 2.0% Ni [5,6]. The plant started in 2013 and during the period 2016–2020 its production was 17–28 kt Ni/y. In Japan, Nippon Yakin is using the Krupp-Renn process, i.e., complete reduction in a R/K for Fe-Ni production with 3–5% Ni [1,14].
3.2. Νickel Pig Iron Production (NPI) with 3–12% Ni
A new quality of ferronickel, Nickel Pig Iron (NPI), with 2.5–5.5% Ni, was produced in China and Indonesia, in 2006 and 2015 respectively, by smelting reduction of laterite in the Blast Furnace (B/F) [15,16]. Nickel pig iron (NPI) has a high C% and Si% due to intensive reduction reactions in the B/F. The proper operation requires feed agglomeration, and coke which is an expensive reductant. NPI is used for the production of a new series No. 200 AISI of austenitic stainless steel (SS) with 3.5–5.5% Ni, which is a lower quality than No. 300 series SS with 8–10% Ni. The R/K-E/F method does not have these disadvantages and for this reason, it has wide application for decades, as shown in Table 4. So, although in 2012–2014 the first B/F unit in Indonesia was started producing 1–2 kt Ni/y in NPI, with 2.25–4.05% Ni, Indonesian new plants, in co-operation with China, are using the R/K-E/F method in NPI production with 10–12% Ni instead of 2.5–5.5% Ni, by the B/F or the E/F methods. In 2016 NPI production with the R/K-E/F method in China and Indonesia reached above 69%, the B/F at about 17% and the E/F at about 14%. These countries moved one step more, by integration of Ni industry with SS production and impressive increase of NPI and SS production, as shown in Figure 1, below.
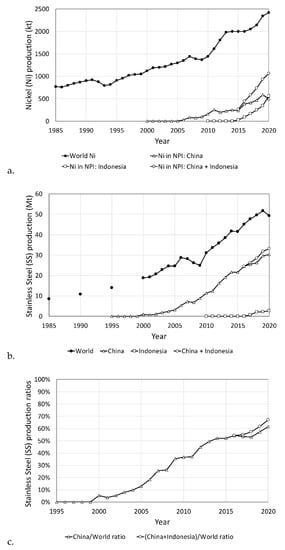
Figure 1.
(a) Nickel production, (b) Stainless Steel production, and (c) Stainless Steel production ratios.
The result of this China (C) and Indonesia (I) co-operation, i.e., (C + I) production from zero of Ni in NPI in 2005 it reached in 2020 at 1060 kt Ni or 43.6% [(=1060/2430.7) × 100] of world primary Ni production. In addition, SS production of China in 2000 was 1000 kt or 5.3% [=(1000/18,800) × 100] of world SS production, and in 2020 it reached 30.3 Mt or 61.5% [=(30,300/49,300) × 100] of world SS production. SS production of both countries in 2000 was 1000 kt or 5.3% [=(1000/18,800) × 100] of world SS production, and in 2020 it reached 33.1 Mt or 67.2% [=(33,126/49,295) × 100] of world SS production, Figure 1. It is also noted that in 2020, Ni from Fe-Ni and NPI used in SS production was 1437.7 kt Ni or 59.1% [=(1437.7/2430.7) × 100] of world primary Ni production and the total Ni contained in SS was 1573 kt or 64.71% [=(1573/2430.7) × 100] of world Ni production. Besides integration of Ni industry in these two countries, they also have a low labor cost, loose environmental laws, and a high Ni content of the Indonesia ore. Therefore, NPI production cost is much lower than Fe-Ni with 20–40% Ni. Integration of Ni industry with SS production is also applied by Posco [8] and Nippon Yakin [14], producers of Fe-Ni. Other plants like PT Vale Soroako, Aneka Tambang, Falcondo, Koniambo, Eramet-SLN and Fenix have their own electric energy production units.
3.3. Production of Ni Matte (Ni3S2)
Production of Ni matte pre-supposes reduction of Fe and Ni oxides to metallic Fe and Ni, and after that reaction of sulfur with Fe and Ni to form an iron and nickel matte (Ni3S2.nFeS). This reduction is necessary because Fe and Ni oxides are thermodynamically more stable than their sulfides [10] (p. 428). Therefore, ore reduction has to take place before Ni matte production. This is applied in Indonesia, at PT Vale Soroako plant, using the R/K-E/F method for more than 40 years. In the feed of R/K is added some source of sulfur, (elemental S or pyrite FeS2 or gypsum CaSO4.2H2O), together with steam coal for reduction, so that metallic Fe and Ni are transformed to sulfides. The R/K calcine is smelted in the E/F for the Ni3S2.nFeS production. The next step is Fe removal and Ni matte, (Ni3S2), production, in a Peirce-Smith convertor (horizontal with side air blowing). Ni3S2 can then be treated for Ni or NiSO4.6H2O production.
Ni3S2 was also produced by using the R/K-E/F method in Guatemala for 3 years (1977–1980) at Exmibal-Fenix plant [1,7]. Since 2014 the plant is producing Fe-Ni with the R/K-E/F method and feed ore with 1.72% Ni [7].
Ni3S2 was also produced in New Caledonia from 1880 until 2016 from Societé Le Nickel, (SLN), by smelting reduction in a B/F, by feeding the ore with coke and some source of sulfur. Removal of Fe from Ni3S2.nFeS was achieved in a Bessemer convertor (with air blowing from the bottom). After Fe removal, Ni3S2 was cast to anodes for electrolysis and metallic Ni production in France. Recently Eramet/SLN stopped Ni3S2 production to give more attention to Fe-Ni production [17].
Ni3S2.nFeS was also produced in Russia from 1938 to 2012 in Southern Urals Nickel Plant, (SUNP), with ore plus carbon agglomeration (sintering) and feeding sinter with pyrite, (FeS2), in a B/F [18], for smelting reduction. The company planned to replace B/Fs with two E/Fs, for Fe-Ni production. The effort started in 2009, but in June 2011 after a fatal accident in the E/F the plant was stopped.
4. The Production of Greek Fe-Ni
4.1. Krupp-Renn and LM-MLar Methods
Greek Fe-Ni industry faced many difficulties for 15 years (1952–1966) until it was able to produce a commercial product. In 1956 the Krupp-Renn process was tried but it was unsuccessful and was abandoned [19,20]. Another 10-year effort (1957–1966) took place by the late professor L. Moussoulos and they gave the LM [21,22] and the MLar [22] methods for the production of metallic Ni 99.9% and steel, respectively, by following the R/K-E/F–convertor(C/V) and electrolysis (EL) steps, and steel production from the slag of the E/F. These methods also failed and were abandoned due to technical and economic problems.
4.2. Larco Method
With Larco method, certain technological and physicochemical modifications of LM process took place, for Fe-Ni production with 20–30% Ni, as follows:
- -
- Refining and enrichment of the E/F Fe-Ni from 15% to 20–30% Ni in a convertor, and not to 95–96% Ni.
- -
- Omitting the electrolysis step.
- -
- Partial reduction of Fe oxides was applied instead of a complete reduction in the R/K.
- -
- Increase of exit opening diameter of the R/K, in order to decrease the retention time of material in the kiln from 10 to 4 h and increase the R/K feeding rate from 10 to 70 t/h.
- -
- Numerous technological and operational improvements were also realized during the 54 years of operation, with new prototype constructions and methods, like e.g., granulation of liquid Fe-Ni [23,24], dimensional analysis of a R/K (LxD = 125 × 6.1 m) with a given capacity and calcine quality [25], the use of dolomitic lime instead of calcite lime for desulfurization and dephosphorization of Fe-Ni, etc. In addition, O2 blowing from the bottom of the convertor (OBM method) and not from the top (LD method), in co-operation with the late professor D. Papamantellos.
- -
- MLar process for steel production was omitted.
The best 11 continuous years of Greek Fe-Ni production were 1994–2004 with a mean yearly Ni production of 17,033 t Ni/y, whereas the mean yearly Ni production of the 54 years of Larco operation (1967–2020) was 13,777 t Ni/y.
4.2.1. Advantages of Larco
- -
- Reliability of the R/K-E/F method and its availability.
- -
- Reliability with all types of laterites.
- -
- Ni metallurgical recovery around 90%.
- -
- Ability to use all types of fuels.
- -
- Larco plant has its own port.
- -
- The R/K-E/F method is used for Ni matte (Ni3S2) production in Indonesia (PT Vale, Soroako), (Table 4) for more than 40 years. Ni3S2 can be used for metallic Ni and NiSO4.6H2O production, materials used for electric vehicle batteries and energy storage.
- -
- Part of Fe in the ore is used in SS production.
- -
- Convertor slag is used for pipe coating, in under the sea oil transportation.
- -
- Part of E/Fs slag is used as a sand blasting material.
4.2.2. Disadvantages of Larco
- -
- The R/K-E/F method is a high thermal and electrical energy consumer.
- -
- The Co contained in laterite is not recovered.
- -
- The lowest quality of Greek laterite among all Fe-Ni producers has a result of very high electric energy consumption (MWh/t Ni) and a very low productivity (wages/t Ni). This fact and other advantages of Fe-Ni producers, like NPI production, economy of scale, integration with SS production, ownership of electric energy units, make Larco’s viability difficult, unless it can be supplied with a better-quality ore.
- -
- The high stripping ratio (overburden waste/ore) of most of Greek Ni ore.
- -
- The excessive electric energy price, imposed very often by a dominant energy supplier.
- -
- The significant amount of CO2/t Ni.
- -
- The very often changes of its administration.
5. Nickel Production by Hydrometallurgical Processing
5.1. Nickel Production by Leaching under Pressure (HPAL)
HPAL method refers to Ni and Co extraction from laterites, with H2SO4 leaching under pressure in autoclaves [1,15,26]. Under the pH conditions in the autoclave, Fe is precipitated as Fe(OH)3, so Fe does not stay in the pregnant solution. This precipitation increases with temperature. The leaching temperature of HPAL is 240 to 270 °C and the pressure 5–45 atm. The final product can be electrolytic Ni, Co, NiO, Ni briquets, a mixture of NiS and CoS, or even a mixture of Ni and Co hydroxides. Method negatives are the high investment and operational cost, and the problem of MgSO4 disposal. In 2020, HPAL was applied at Moa Bay in Cuba, at Glencor Murrin-Murrin in Australia, at Ambatovy plant in Madagaskar, and at Vale VNC Goro in New Caledonia. Goro started in 2009 with average production from 2016 to 2020 of 28.4 kt Ni/y but after many administrative and technical difficulties, this year (2021) media reported that Goro stopped its production. The companies Bulong, Cawse, and BHP Billiton Ravensthorp in Australia were not in operation in 2020.
5.2. Nickel Production by Atmospheric Leaching, (AL) and Heap Leaching, (HL)
Atmospheric leaching, (AL), of laterite is leaching with H2SO4 in atmospheric conditions and up to 100 °C, and heap leaching is leaching with H2SO4 in heaps (HL). Both methods were always a challenge because of the low investment and operational cost. For this reason, there have been numerous studies, research work and industrial efforts for decades. Recently, 82 kt Ni were produced by AL process, from 2007 to 2014, and 72 kt Ni by HL from 2006 to 2016. The high market demand for Ni and NiSO4.6H2O in electric vehicles and other applications during the last few years has changed the prospects of Ni laterite hydrometallurgical processing and it has already increased the price of these Ni products, thus today a premium above LME price is paid for them, whilst ferronickel is sold with discounts. Additional substantial advantages of the method are the very low CO2 emissions as well as low electricity consumption, plus the additional Cobalt product, which comes at a very high price, today being three times the price of nickel.
Hellenic Minerals Ltd. in Cyprus, after two years of successful industrial testing, is in the commissioning state of a Heap Leaching-Solvent Extraction-Crystallizer (HL-SX-CR) unit for NiSO4.6H2O production. The plant capacity is 10 kt Ni/y and 200 t Co/y.
6. NiO Production, by Pyro- and Hydro-Metallurgical Treatment. Caron Method
Caron method is the combination of pyro- and hydrometallurgical processing of laterite. That is, reduction roasting and then calcine leaching with ammonia, for NiO production. Direct ammonia, (NH3), leaching of Ni oxides is impossible, whereas metallic Ni is dissolving in NH3. So, selective reduction of Ni oxides at 700–750 °C is taking place, and then ammonia, (NH3), leaching of the calcine [1,2,20]. Above 750 °C the hydrosilicate minerals of Mg, keep NiO in their structure, and they are not leached by NH3, decreasing Ni and Co recovery. So, this method can be only applicable to limonites with low Mg. This method or its variants have been applied from the companies Nicaro and Punda Corda in Cuba, Yabulu (Greenvale) in Australia, Tocantins in Brazil and Nonoc (Marinduque) Surigao in the Philippines, but today they are not in operation and according to bibliography (2009), the Caron method “is considered unlikely to be used in the future” [27].
7. Nickel Production for Batteries
Environmental concern and the relative laws have driven towards Ni batteries for electric vehicles and energy storage. So, the effort to reduce atmospheric pollution has increased electric vehicles (EVs), from zero in 2000, to 2 million in 2018, to 3 million in 2020, and the forecast is 14 million in 2025, and 31 million in 2030. Environmental laws are the main factor leading to EVs. For example, CO2 emissions in 2017 of EU cars were 118 g/km, of US 155 g/km, and of China 179 g/km, and the forecast for 2030 is for EU 62 g/km and for US 85 g/km. Metallic Ni and NiSO4.6H2O are used for electric vehicle batteries and for energy storage, and the Ni demand of these materials is expected to reach from 140 kt Ni in 2018, to 1100 kt Ni in 2030 [28] (p. 17). In addition, lithium-ion batteries are expected to increase Ni demand by more than 5.2 times during the 2021–2030 period [29].
8. Conclusions
From this work it comes that the world’s primary nickel production in 2020 was 2430.7 kt Ni; 69% (1677.7 kt) of this production was from oxidized nickel ores (laterites) and 31% from sulfides. Eighty-seven point seven percent (87.7%) of Ni produced from laterites was by pyrometallurgical and 12.3% by hydrometallurgical processes.
For decades Fe-Ni analysis was 20–40% Ni, but in 2006 a new Fe-Ni quality came into the scene. This was the nickel pig iron (NPI) with 2.5–5.5% Ni coming from smelting reduction of laterite in the blast furnace (B/F). In a short time, the advantages of R/K-E/F process led to its dispersion in China and Indonesia and resulted in an NPI production with 10–12% Ni. So, NPI production in these two countries climbed from zero kt Ni in 2000, to 1060 kt in 2020, and stainless steel (SS) production reached from 5.5% in 2000 to 67.2% in 2020 of the world SS production. China alone achieved 61.5% of world SS production in 2020. The advantages of NPI and SS production were the integration of Ni industry with SS production, the economy of scale, the low labor cost, the high Ni content of Indonesian laterite, and the loose environmental laws, and they reduced significantly the NPI production cost. The addition of SS and/or electric power production units, to reduce the production cost, has been also adapted by other Fe-Ni producers.
On the other hand, although global Ni production was doubled in the last 20 years, it is expected to increase even more (from 140 kt Ni in 2018 to 1100 kt Ni in 2030), due to the use of Ni and NiSO4.6H2O in electric vehicles and energy storage. Hellenic Minerals in Cyprus after two years of successful industrial tests is in the commissioning state of a Heap Leaching-Solvent Extraction-Crystallizer (HL-SX-CR) unit for NiSO4.6H2O production. The high demand for Ni and NiSO4.6H2O in the last few years has changed the prospects of Ni laterite hydrometallurgical processing, thus poor Ni laterite resources are expected to be attractive for new hydrometallurgical investments, since they make economic value, which is not the case when it comes to pyro-metallurgical processes.
Regarding the R/K-E/F method used in Greek Fe-Ni, it is characterized by its worldwide acceptance and reliability (more than 77% of world Fe-Ni production comes from this process). Other advantages are the use of all types of laterites and fuels, it has a high metallurgical recovery, the plant has its own port, part of the slags is used in other applications, and its Fe is used in SS production. However, it is a high energy-consuming process and it does not recover Co. The Greek laterite, in particular, has the lowest Ni% among all Fe-Ni producers and because of this, electric energy (MWh/t Ni) and wages (wages/t Ni) per ton of Ni are high, making Larco’s viability difficult, unless it can be supplied by a better-quality ore. Other negatives were the excessive electric energy price it had to pay to a dominant energy supplier, and the very frequent changes of its administration. Finally, in order to keep the Greek laterites at play, the solution is to combine pyro-metallurgy for ferronickel production and in parallel to introduce hydrometallurgy for Ni and NiSO4.6H2O production, both processes requiring ore blends of domestic laterites and imported high grade Ni ores, in order to achieve best economic results.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dalvi, A.D.; Bacon, W.G.; Osborne, R.C. The Past and the Future of Nickel Laterites. In Proceedings of the PDAC 2004 International Convention, Trade Show & Investors Exchange, Inco Limited, Mississauga, ON, Canada, 7–10 March 2004. [Google Scholar]
- Duyvesteyn, W.P.C.; Wicker, G.R.; Doane, R.E. An Omnivorous Process for Laterite Deposits. In Proceedings of the International Laterite Symposium, New Orleans, LA, USA, 19–21 February 1979; Evans, D.J.I., Shoemaker, R.S., Veltman, H., Eds.; AIME: Warrendale, PA, USA, 1979; pp. 553–570. [Google Scholar]
- Alcock, R.A. The Character and Resources Available to the Nickel Industry. In Proceedings of the Symposium of Extractive Metallurgy of Nickel and Cobalt: Proceedings of a Symposium, 117th TMS Annual Meeting, Phoenix, AZ, USA, 25–28 January 1988; pp. 67–89. [Google Scholar]
- Warner, A.E.M.; Diaz, C.M.; Dalvi, A.D.; Mackey, P.J.; Tarasov, A.V. JOM World Nonferrous Smelter Survey, Part III: Nickel: Laterite. JOM 2006, 58, 11–20. [Google Scholar] [CrossRef]
- Moutafis, I. (General Manager, Falcondo, Bonao, Dominican Republic). Technical report from visit to Koniambo, Falcondo, New Caledonia. Private communication, 2014; Unpublished work. [Google Scholar]
- Mining Technology. Koniambo Nickel Project. 25 November 2014. Available online: https://www.mining-technology.com/projects/koniambo-nickel-project/ (accessed on 17 July 2021).
- Tavchandjian, O.; Golightly, P. Technical Report on an Update to the Fenix Project, Izabal, Guatemala. 31 March 2010. Available online: https://www.sec.gov/Archives/edgar/data/1322422/000119312510231823/dex9973.htm (accessed on 7 July 2021).
- Posco-SMSP (Societé Minier South Pacific). Nickel. Joint Venture Agreement. 5 April 2008. Available online: https://www.POSCO-smsp.nc (accessed on 7 July 2021).
- Raw and Intermediate Materials, News and Views. Battery Mater. Rev. 2021, 4, 8.
- Zevgolis, E. Ferronickel (Fe-Ni)-Chapter 12. In Iron Metallurgy, Theory and Technology, 1st ed.; IWN: Athens, Greece, 2014; pp. 409–534. (In Greek) [Google Scholar]
- Anglo American. Annual Report-Nickel. Available online: https://www.angloamerican.com (accessed on 27 June 2021).
- Zevgolis, E.; Daskalakis, K.; Gjorgjiev, D. Sustainable Development in the Mining and Metallurgical Industry. The Case of FENI Industries. Available online: https://www.researchgate.net/publication/321010603_Sustainable_Development_in_the_Mining_and_Metallurgical_IndustryThe_Case_of_FENI_Industries (accessed on 15 July 2021).
- Zevgolis, E. Report from visit to Falcondo. Bonao, Dominican Republic, 27.04.2015–1.5.2015. Unpublished work.
- Watanabe, T.; Ono, S.; Arai, H.; Matsumori, T. Direct reduction of garnierite ore for production of ferro-nickel with a rotary kiln at Nippon Yakin Kogyo Co., Ltd., Oheyama Works. In Proceedings of the International Seminar on Laterite, Tokyo, Japan, 14–17 October 1985; pp. 103–115. [Google Scholar]
- British Geological Survey. Nickel. Definition, Mineralogy and Deposits. September 2008. Available online: www.mineralsUK.com (accessed on 27 June 2021).
- McRae, M.E. Nickel. US Geological Survey, Minerals Yearbook-2016, National Minerals Information Center, Nickel Statistics and Information. Available online: https://www.usgs.gov (accessed on 13 May 2021).
- Eramet, S.A. SLN Focuses on 25 Ferronickel Production. 2016. Available online: https://www.eramet.com/en/sln-focuses-slnr-25-ferronickel-production (accessed on 28 May 2021).
- Zevgolis, E. Report from visit to SUNP (Southern Urals Nickel Plant). Orsk, Orenburg, Russia. 23 January 2013; Unpublished work. [Google Scholar]
- Zevgolis, E. The Greek Ferronickel and its Contribution to the Economic and Technological Development of Greece. In Proceedings of the “Scientific Contribution for the 50 years of the Mining and Metallurgical Engineering Department” Conference, Athens, Greece, 3–4 March 1997. (In Greek). [Google Scholar]
- Moussoulos, L. Nickel Metallurgy; National Technical University of Athens: Athens, Greece, 1973; pp. 185–200. (In Greek) [Google Scholar]
- Moussoulos, L. The L.M. Process Extraction of Nickel and Cobalt from Laterites. In TMS of AIME, Paper Selection, Paper No. A71-4; AIME: Warrendale, PA, USA, 1971. [Google Scholar]
- Moussoulos, L. Extraction of Nickel, Cobalt, Iron and Chromium from Laterites. L. M. and M-LAR Processes. In Proceedings of the Symposium: The Institution of Mining and Metallurgy, London, UK, 17–20 April 1967. [Google Scholar]
- Zevgolis, E.; Argyriou, S.; Redizelas, G. A Granulation Method of Liquid Metal. Patent No. 59964, 21 April 1978. (In Greek). [Google Scholar]
- Zevgolis, E. The Granulation of Ferronickel and Ferrochromium. In Mining and Metallurgical Annals; Society of Mining and Metallurgical Engineers: Athens, Greece, 1995; Volume 5, pp. 9–22. (In Greek) [Google Scholar]
- Zevgolis, E. Dimensional Analysis of Rotary Kilns in Nickeliferous Laterite Reduction. In Honorary Volume for NTUA Professor Emeritus L. Moussoulos; Department of Mining and Metallurgical Engineering, National Technical University of Athens: Athens, Greece, 1992; pp. 77–97. (In Greek) [Google Scholar]
- Department of Resources Development. In Nickel Review; Department of Resources Development: Perth, Australia, 1999.
- Wedderburn, B. Nickel Laterite Processing—A shift towards heap leaching. ALTA. In Proceedings of the Nickel-Cobalt Conference, Perth, Australia, 25–27 May 2009; ALTA Metallurgical Services: Melbourne, Australia, 2009. [Google Scholar]
- Hellenic Minerals Ltd. Cyprus Brochure; Hellenic Minerals Ltd.: Egkomi, Cyprus, 2021; pp. 1–21. [Google Scholar]
- Mining.com. Chart: Study Predicts over 400% Increase in Cu, Li, Ni, Battery Demand, Editorial, 30 June 2021. Available online: https://www.mining.com/chart-study-predicts-over-400-increase-in-copper-lithium-nickel-battery-demand/ (accessed on 15 August 2021).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).