Abstract
Improved corrosion protection of low-carbon steel was achieved by barrier non-toxic sol–gel multilayered structures composed of a ZrO2 top coating and TiO2 underlayers. The zirconium precursor solution was maintained as constant, whereas the TiO2 solution composition was modified with two different types of polymers, which were added separately to the starting titanium solution. The phase composition, morphology and corrosion protective properties were analyzed by X-ray diffraction spectroscopy (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM) and atomic force microscopy (AFM). Hydrophobicity properties were evaluated by measuring the contact angle with a Ramé-Hart automated goniometer. The potentiodynamic polarization technique was used to determine the corrosion resistance and protective ability of the coatings in a 5% NaCl solution. Both polymeric modifications, as compared to the non-modified titania layer, demonstrated positive effects on the corrosion properties of the structures at conditions of external polarization. Due to the amorphous structure of the zirconia layer and its relatively dense, hydrophobic surface, all of the samples extended the service life of low-carbon steel in a model corrosion medium. The feasibility of the sol–gel deposition method makes it possible to prepare oxide coatings with appropriate surface properties, which ensures high corrosion resistance.
1. Introduction
Low-carbon structural steels are widely used in the chemical and civil engineering fields due to their advantages of low cost, manufacturing flexibility and availability. Carbon steels have ferrite–perlite microstructures and usually contain C and Mn (Mn up to 2 wt%, C from 0.02 to 0.5 wt% and Si up to 0.5 wt%). During their operation, these materials are often subject to corrosion attack as a result of their interaction with surrounding media, as well as low overvoltage of hydrogen and/or low overvoltage of the ionization process on the iron surface. This leads to an accelerated corrosion process and the faster destruction of the latter. The presence of oxidants in the surrounding media has a strong influence on the corrosion potential value and on the corrosion rate of the iron. For example, in slightly acidic and neutral aqueous solutions, low soluble by-products of corrosion (well known in everyday practice as “rust”) are formed. Some of them have weak protective properties, but others can successfully defend the substrate for a certain period of time. Generally, low-carbon steels (mild steels) are distinguished by not-very-well-expressed corrosion resistance. That is the reason to look for and to apply appropriate means of corrosion protection (for example, inhibitors, coatings etc.) during their operation at different conditions.
Various types of protective coatings have been developed to enhance the life span of carbon steel products such as organic monolayer [1], glass coatings [2] and ceramic coatings [3]. Nanosized oxide coatings possess good thermal and electrical properties, as well as higher resistance to oxidation, corrosion and wear than metals. Recently, the corrosion resistance of TiO2 [4,5], SiO2 [6], Al2O3 [7] and ZrO2 [3] coatings have been studied. A variety of synthesis methods, such as the sol–gel method, hydrothermal method, direct oxidation method, electrodeposition, physical vapor deposition etc., have been developed for the synthesis of TiO2 nanostructures, and their anti-corrosion properties have been investigated [8,9,10]. Among them, the sol–gel method has many advantages; it offers easy control of the microstructure and requires low-cost equipment, and it is a waste-free method, which is environmentally and economically more acceptable than the other deposition methods. Zirconium dioxide coatings are widely used in different areas, and especially as barrier anticorrosive coatings, due to their excellent physical and chemical properties [11]. The low cost, high hardness, chemical stability and good optical, photo-electrochemical and protective properties of TiO2 coatings provide their effectiveness in various technological areas [12]. The common advantages of both oxides are their non-toxicity and biocompatibility. The aim of this study is to obtain environmentally-friendly TiO2/ZrO2 multilayers, deposited on low-carbon steel using the waste-free sol–gel method, that exhibit high corrosion resistance.
2. Materials and Methods
Three types of multilayer titania–zirconia protective coating systems, each of which consisted of 3 underlayers of TiO2 (sequentially applied) and a top single layer of ZrO2, were deposited by the sol–gel method on low-carbon steel substrates (C—0.05–0.12; S ≤ 0.04; P ≤ 0.35; Mn—0.25–0.5; Cr ≤ 0.1; Si ≤ 0.03; Ni ≤ 0.3; Cu ≤ 0.3; As ≤ 0.08; Fe-balance). The substrates were cleaned ultrasonically in ethanol before deposition. Titania-based coatings were prepared from three different types of sols. Sol A contained titanium tetrabutoxide (TB), which was dissolved in isopropanol and acetacetone (0.4 M/l). Sol B also contained a small quantity of polymeric modificator polyoxyethylene (20) sorbitan monooleate (Tween 80), while Sol C contained ethylcellulose. Zirconium-based coatings were obtained from Zr butoxide; Zr(OC4H9)4 and a small quantity of nitric acid were dissolved in 2-propanol. Acetic acid and acetylacetone were added as complexing agents. Finally, 5 wt% polyethylene glycol (Mw = 400) was added as a structure directing agent. The substrates were dipped in each of sols A, B and C and were withdrawn at a rate of 30 mm/min. After each deposition, the samples were dried consecutively at 100 °C and 200 °C for 1 h. The dipping–drying procedure was repeated three times. Then the deposits were dipped into zirconium solution. The final treatment of the samples was carried out at 500 °C with a heating rate of 5 °C/min. At the end of this process, sample A consisted of 3 underlayers of TiO2 obtained from Sol A; sample B, of 3 underlayers of TiO2 obtained from Sol B; sample C, of 3 underlayers of TiO2 obtained from Sol C. As mentioned above, all these systems were finished with a top single layer of ZrO2. The phase compositions of the samples were studied by X-ray diffraction (XRD) with CuKα-radiation (Philips PW 1050 apparatus). The surface topography was studied by means of an atomic force microscope (AFM) (NanoScopeV system, Bruker Inc., Billerica, MA, USA). The scanning rate was set at 1 Hz. Subsequently, all the images were flattened by means of the Nanoscope software. The chemical composition and morphology were studied by SEM/EDX analyses (JEOL JSM 6390 and INCA Energy 350 unit, Tokyo, Japan). X-ray photoelectron spectroscopy (XPS) was applied to investigate the chemical composition and electronic structure of the films’ surfaces. The measurements were carried out on an AXIS Supra electron spectrometer (Kratos Analytical Ltd., Manchester, UK) using achromatic AlKα radiation with a photon energy of 1486.6 eV and a charge neutralization system. The binding energies (BE) were determined with an accuracy of ±0.1 eV, using the C1s line at 284.6 eV (adsorbed hydrocarbons). The chemical composition in the depth of the films was determined by monitoring the areas and binding energies of C1s, O1s, Ti2p and Zr3d photoelectron peaks. Using the commercial data-processing software of Kratos Analytical Ltd., the concentrations of the different chemical elements (in atomic %) were calculated by normalizing the areas of the photoelectron peaks to their relative sensitivity factors. Contact angle measurements were performed with a Ramé-Hart automated goniometer model 290 with DROPimage advanced v2.4 (Succasunna, NJ, USA) at room temperature. Drops of 2–5 μL were formed and deposited with a Ramé-Hart automatic dispensing system. The anticorrosion and protective properties of the multilayer systems were analyzed using the potentiodynamic polarization test. The curves were performed for characterization of the corrosion process and protective properties as well as for evaluation of the corrosion behavior of the multilayer coating systems at conditions of external polarization in the model test medium using a VersaStat 4 (PAR) unit (Princeton Applied Research, Oak Ridge, TN, USA). The investigations were carried out in a three-electrode electrochemical cell of 250 mL volume at a scan rate of 1 mV/s. The counter-electrode was platinum wire, and a saturated calomel electrode (SCE) was used as a reference electrode. Prior to the start of the experiment, the samples were kept in the model medium at open circuit potential (OCP) conditions for 15 min in order to stabilize their corrosion potential values. The investigations were held in a model corrosion medium of 5 wt% NaCl solution at pH 6.7 and at ambient temperature. The reproducibility of the realized investigations was an average of 5 samples per sample type.
3. Results and Discussion
3.1. Phase Structure
The presence of two compounds/iron oxides, magnetite and hematite phases, were registered by XRD analyses of all of the coating (Figure 1). This result was probably due to the oxidation of the substrate during the thermal treatment. Nevertheless, the peak that belongs to the anatase phase of TiO2 is well visible, which is due to the type of the multilayer system consisting of three layered TiO2 coatings. It could be supposed that the zirconia layer possessed an amorphous structure, because zirconia crystallographic phases were not registered. Several research groups have also revealed that the amorphous phase of the composites on the base of TiO2 and ZrO2 is stable after treatment to 450 °C [13] and even at 600 °C [14].
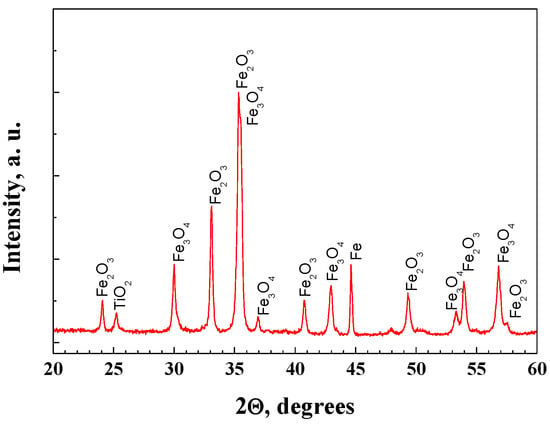
Figure 1.
Typical XRD pattern of multilayer structure A.
3.2. Surface Morphology
The SEM images of the investigated structures reveal relatively homogeneous dense morphology without visible cracks A few secondary small crystalline particles were formed on the surface (Figure 2). Similar observations can be made from the AFM images in Figure 3.
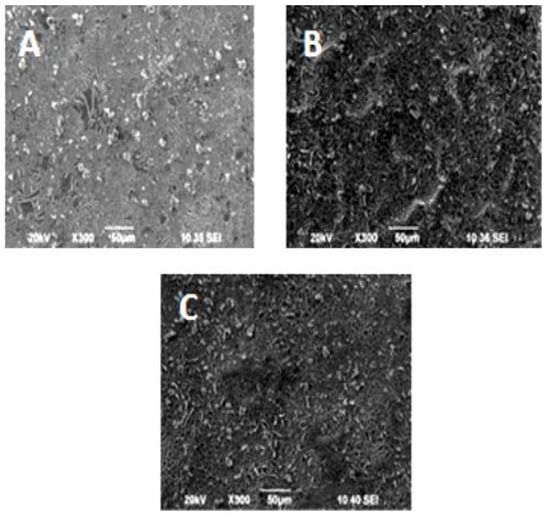
Figure 2.
SEM photographs of the multilayer structures: (A) 3 layers TiO2 (sol A) and top ZrO2 layer; (B) 3 layers TiO2 (sol B) and top ZrO2 layer; (C) 3 layers TiO2 (sol C) and top ZrO2 layer.

Figure 3.
AFM 2D images of multilayer structures A (left) and C (right).
3.3. XPS Analyses of the Surface Composition
The XPS results show that peaks of C1s, O1s, Zr3d, Ti2p and Fe2p were registered on the surface. The oxygen spectra consist of three components. The first one, at 530 eV, corresponds to oxygen bound to metals, while the second and third ones, at 531.6 eV and 533.4 eV respectively, are attributed to adsorbed hydroxyl groups and surface water (Figure 4). The zirconium spectra are decomposed into several peaks associated with ZrO2 (with binding energies 182.7 eV for Zr3d5/2 peaks and 185.2 eV for Zr3d3/2 peaks) and ZrOH (with binding energies 184.0 eV for Zr3d5/2 and 186.7 eV for Zr3d3/2). 1.4 at% TiO2 and 13 at% Fe2O3 are also registered on the surface. From the areas of the deconvoluted oxygen peaks, it can be concluded that the amount of hydroxyl groups is the largest in multilayer C.
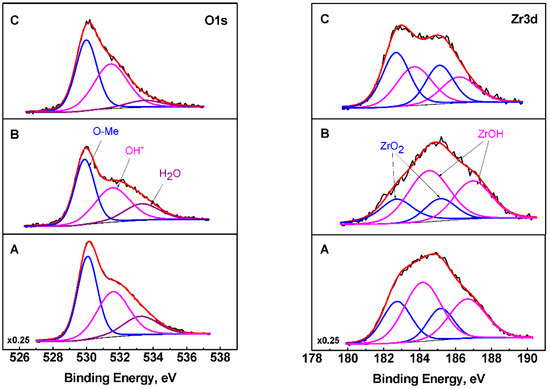
Figure 4.
O1s and Zr3d core level spectra of the multilayered structures after deconvolution, where: (A) 3 layers TiO2 (sol A) and top ZrO2 layer; (B) 3 layers TiO2 (sol B) and top ZrO2 layer; (C) 3 layers TiO2 (sol C) and top ZrO2 layer.
3.4. Contact Angle Measurements
The measurement of the contact angles of the layers revealed that all of the studied multilayers exhibited hydrophobic properties (Figure 5). It has to be noted that the contact angle value is not dependent on the composition of the titania precursor solution.
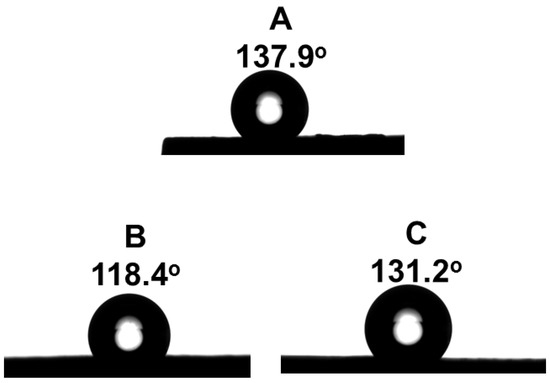
Figure 5.
Contact angles of the multilayer structures. (A) 3 layers TiO2 (sol A) and top ZrO2 layer; (B) 3 layers TiO2 (sol B) and top ZrO2 layer; (C) 3 layers TiO2 (sol C) and top ZrO2 layer.
3.5. Corrosion and Anodic Behavior of the Structures Evaluated by Potentiodynamic Curves
The PD curves of the multilayer structures are demonstrated in Figure 6. It is well visible that all the systems had close corrosion potentials (between −0.605 V and −0.611 V, respectively), which could be expected as a result of their nature and composition. More interesting is the anodic branch of the curves. The registered corrosion current densities of the investigated systems were as follows: for multilayer A, 8.1 × 10−6 A/cm−2; for multilayer B, 4.3 × 10−6 A/cm−2; for multilayer C, 2.3 × 10−6 A/cm−2. In addition, as a result of the applied external anodic polarization, multilayer A presented higher anodic current densities compared to B and C (up to about one order) in the whole potential area during the investigation. These observations led to the conclusion that the application of some selected polymers positively affected the corrosion resistance properties of the sol–gel coatings in that medium, ensuring their lower current densities in the anodic region.
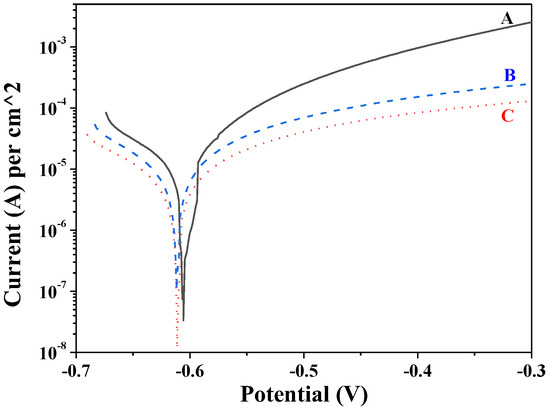
Figure 6.
Potentiodynamic polarization curves of the multilayers in the model test medium.
The oxide layer structures investigated in this paper exhibited enhanced resistance in a corrosive salt medium due to their better protective properties compared to monolayer coatings. This type of structure combined the advantages of both single oxides. This includes the good barrier properties, adhesion to steel substrate [15] and high electrical resistivity and mechanical parameters of the TiO2 layers [16]. On the other hand, the ZrO2 top coating possessed high corrosion and wear resistance as well as chemical stability [3]. In addition, the amorphous structure of the outer zirconia layer deteriorated ion and electron conduction, thus retarding cathodic or anodic electrochemical processes due to inhibition of intergranular corrosion between the grains (IGC) [17]. According to literature, the presence of the amorphous structure of titania-based composite films improves protective properties [18].
Similar results were presented by Holgado et al., which proposed that the corrosion-protective effect of the ZrO2 coatings was due to their high density and partial amorphous character of the films [19]. It must be kept in mind that the relatively dense, amorphous structure combined with the hydrophobic properties of the multilayers obtained induced higher anticorrosion behavior.
4. Conclusions
Multilayer titania/zirconia coatings, where three different titanium precursor solutions were used for TiO2 layers, were coated over low-carbon steel by a sol–gel dip-coating method. Surface and microstructural analysis confirmed the presence of zirconium and titanium oxide over the substrate. The coatings were amorphous with relatively dense surface morphology. The anti-corrosion properties of the multilayer coating systems were evaluated using 5% NaCl solution as a model medium. The polymer addition into titania solution led to the formation of multilayer structures with lower current densities in the anodic region, ensuring better anti-corrosion performance than those consisting of non-modified TiO2 underlayers. It could be supposed that the good protective properties of the multilayers were due to their amorphous, dense surfaces and hydrophobic nature.
Author Contributions
Conceptualization, I.S. and D.S.; methodology, I.S. and D.S.; validation, I.S., D.S, N.B. and N.S.B.; investigation, I.S., D.S., N.S.B., N.B., M.S., S.S. and N.G.; writing—original draft preparation, I.S., D.S. and N.B.; supervision, I.S., D.S. and N.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Bulgarian FUND FOR SCIENTIFIC RESEARCHES at the Ministry of Education and Science, PROJECT No. KP-06-H37/16 “New environmentally friendly one- and multi-layer coatings for corrosion protection of structural materials with wide application”. The authors express their gratitude for the financial support given to realize the investigations.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are acknowledged to the contract “Mono-and poly-component catalytic systems for waste water and polluted air purification from model contaminants”, which is within the Non-currency Equivalent Exchange Bilateral Cooperation between the Bulgarian Academy of Sciences and the Serbian Academy of Sciences and Fine Arts.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meth, S.; Savchenko, N.; Koltypin, M.; Starosvetsky, D.; Groysman, F.A.V.A.; Sukenik, C.N. Corrosion studies of stainless steel protected by a TiO2 thin film deposited on a sulfonate-functionalized self-assembled monolayer. Corros. Sci. 2010, 52, 123–129. [Google Scholar] [CrossRef]
- Fu, G.; Wei, L.; Chang, X.; Cui, Y.; Wang, Y.; Yu, B.; LV, C.; Ye, S. A MgO–SiO2–Al2O3–ZnO Ceramic-glass Coating to Improve the Anti-oxidation of Carbon Steel at High Temperature 2018. ISIJ Int. 2018, 58, 929–935. [Google Scholar] [CrossRef]
- Stambolova, I.; Dimitrov, O.; Yordanov St Blaskov, V.; Vassilev, S.; Shipochka, M.; Boshkov, N. Preparation of newly developed CeO2/ZrO2 multilayers: Effect of the treatment temperatutre on the structure, and corrosion performance of stainless steel. J. Alloys Comp. 2019, 806, 1357–1367. [Google Scholar] [CrossRef]
- Yordanov St Stambolova, I.; Lakov, L.; Jivov, B.; Blaskov, V.; Vassilev, S. Efect of CeO2 dopant on the structure and protection properties of TiO2 sprayed coatings deposited on carbon steel. J. Chem. Technol. Metall. 2018, 53, 1179–1185. [Google Scholar]
- Yordanov, S.T.; Lakov, L.; Stambolova, I.; Blaskov, V.; Djakova, V.; Eliyas, A. Surface morphology and corrosion resistance of neodimum doped TiO2. Sol-gel coatings. Comptes Rendus de l’Académie Bulgare Sci. 2018, 71, 619–621. [Google Scholar]
- Yu, Q.; Ma, X.H.; Wang, M.Z.; Yu, C.J.; Bai, T. Influence of embedded particles on microstructure, corrosion resistance and thermal conductivity of CuO/SiO2 and NiO/SiO2 nanocomposite coatings. Appl. Surf. Sci. 2008, 254, 5089–5094. [Google Scholar] [CrossRef]
- Feng, Q.Y.; Li, T.J.; Teng, H.T.; Zhang, X.L.; Zhang, Y.; Liu, C.S. Investigation on the corrosion resistance and oxidation of Ni-Al2O3 nano-composite coatings by sediment co deposition. Surf. Coat Technol. 2008, 202, 4137–4144. [Google Scholar] [CrossRef]
- Yun, H.; Lin, C.J.; Li, J.; Wang, J.R.; Chen, H.B. Low-temperature hydrothermal formation of a net-like structured TiO2 film and its performance of photogenerated cathode protection. Appl. Surf. Sci. 2008, 255, 2113–2117. [Google Scholar] [CrossRef]
- Ohko, Y.; Saitoh, S.; Tatsuma, T.; Fujishima, A. Photoelectrochemical anticorrosion and self-cleaning effect of a TiO2 coating for type 304 stainless steel. J. Electrochem. Soc. 2001, 148, B24–B28. [Google Scholar] [CrossRef]
- Karpakam, V.; Kamaraj, K.; Sathiyanarayanan, S. Electrosynthesis of PANI-nano TiO2 composite coating on steel and its anti-corrosion performance. J. Electrochem. Soc. 2011, 158, C416–C423. [Google Scholar] [CrossRef]
- Xia, W.; Li, N.; Deng, B.L.; Zheng, R.; Chen, Y. Corrosion behavior of a sol-gel ZrO2 pore-sealing film prepared on a micro-arc of aluminum alloy. Ceram. Intern. 2019, 45, 11062–11067. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Lin, Y.S. Structural and surface chemical properties of sol–gel derived TiO2–ZrO2 oxides. Appl. Catal. A 2004, 265, 35–42. [Google Scholar] [CrossRef]
- Hu, M.; Payzant, Z.C.; Booth, E.A.; Rawn, K.R.; Hunt, R.D.; Allard, L.F. Ultrafine microsphere particleszirconium titanate produced by homogeneous dielectric-tuning coprecipitation. J. Mater. Sci. 2003, 38, 3831–3844. [Google Scholar] [CrossRef]
- Schiemann, D.; Alphonse, P.; Taberna, P.-L. Synthesis of high surface area TiO2 coatings on stainless steel by electrophoretic deposition. J. Mater Res. 2013, 28, 2023–2030. [Google Scholar] [CrossRef]
- Jh Dhiflaoui, H.; Kaouther, K.; Larbi, A.B.C. Wear Behavior and Mechanical Properties of TiO2 Coating Deposited Electrophoretically on 316 L Stainless Steel. J. Tribol. 2018, 140, 031603. [Google Scholar] [CrossRef]
- Kadhum, A.H.; Mohamad, A.B.; Hammed, L.A.; Al-Amiery, A.A.; San, N.H.; Musa, A.Y. Inhibition of mild steel corrosion in hydrochloric acid solution by new cumarin. Materials 2014, 7, 4335–4348. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, T.; Shahrabi, A.A.; Oskuie, H.H.; Sanjabi, S. Effect of heat treatment on corrosion properties of of sol–gel titania–ceria nanocomposite coating. J. Alloys Comp. 2010, 504, 237–242. [Google Scholar] [CrossRef]
- Holgado, J.P.; Pérez-Sánchez, M.; Yubero, F.; Espinós, J.P.; González-Elipe, A.R. Corrosion resistant ZrO2 thin films prepared at room temperature by ion beam chemical vapor deposition. Surf. Coat. Technol. 2002, 151–152, 449–453. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).