Abstract
Phosphate diesters and plasmid DNA are cleaved by gold nanoparticles functionalized with Zn(II)-triazacyclonononane complexes with different mechanisms, dinuclear and mononuclear, respectively, with impressive rate accelerations with respect to the uncatalyzed processes.
1. Introduction
Despite being the fundament of life for living organisms, many functionalities of DNA and RNA still present as challenging targets for scientists. One such aspect is the elucidation of the complete mechanism behind the hydrolysis of the nucleic acids’ backbone, comprised of repeated phosphodiester bond segments linking adjacent nucleosides. Indeed, the phosphate bond is known for its superb stability [1], which allows RNA and DNA to remain intact at 25 °C, pH 7.0 for hundreds and millions of years, respectively. However, enzymes called nucleases perform phosphodiester cleavage in seconds, a result yet to be surpassed by artificial competitors [2].
To date, not even the most meticulously designed molecules have approached the efficiency demonstrated by nucleases, and researchers are still trying to understand the underlying hydrolytic mechanisms behind these enzymes [3]. Accordingly, we decided to challenge ourselves with that issue, and by using the principles of nano and supramolecular chemistry, we attempted to reach enzymes’ efficacy and elucidate the mechanism of phosphodiester cleavage [4].
2. Experimental Details
The details of the synthesis and characterization of the ligands reported in Figure 1 and the gold nanoparticles obtained by passivation of ca. 2 nm gold clusters have been reported elsewhere [5,6].
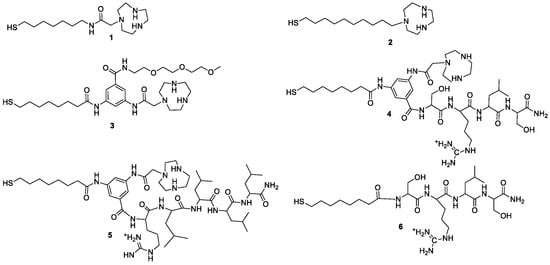
Figure 1.
Chemical structure of the thiolated molecules 1–6 used for the passivation of AuNPs.
3. Cleavage of Bis(p-nitrophenyl) Phosphate (BNP) by AuNPs-Zn(II) Complexes
BNP, being a phosphate diester often used as a DNA model substrate, was chosen for a preliminary screening of our catalysts collection. The reported rate constant for the spontaneous hydrolysis of BNP is very slow (kH2O = 2 × 10−10 s−1 at 50 °C) [7]. In the presence of AuNP1-3 and a stoichiometric amount of Zn(II) (AuNP1-3-Zn(II)), there is a substantial increase in the rate of cleavage. In comparison with the OH-catalyzed process, the three catalysts provided accelerations of 54,166, 14,583, and 37,083, respectively. The hydrolysis stops after the release of a single p-phenolate with the only formation of the phosphate monoester p-nitrophenyl phosphate (Figure 2). AuNP4-Zn(II) and AuNP5-Zn(II) were not particularly soluble in an aqueous solution in the presence of the very lipophilic substrate BNP. The problem was solved in part for AuNP4-Zn(II) by adding 5% DMSO to the solution, but not at all for AuNP5-Zn(II). Accordingly, the latter could not be studied due to the immediate precipitation of the highly lipophilic complex these nanoparticles form with BNP. The data indicated that AuNP4-Zn(II) was a rather poor catalyst. The order of activity of the different nanoparticles was hence AuNP1-Zn(II) > AuNP3-Zn(II) > AuNP2-Zn(II) >> AuNP4-Zn(II). The analysis of the kinetic data indicated that the hydrolysis of BNP catalyzed by the gold nanozymes requires a dinuclear catalytic site that is easily achieved within the monolayer passivating the gold nanoparticles [5,8,9].
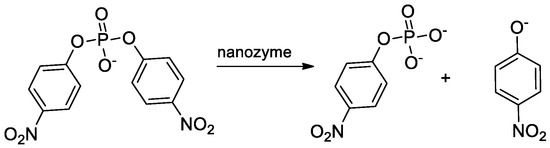
Figure 2.
Hydrolysis of BNP by gold nanozymes.
4. Cleavage of Plasmid DNA by AuNPs-Zn(II) Complexes
The plasmid pBR322 is a circular double-strand DNA, 4361 base pairs (bp) in length. The cleavage of one single phosphodiester bond transforms the native supercoiled form (form I) into the nicked circular one (form II). This latter, if further cleaved within ca. 15 nucleobases in the complementary strand, is transformed into the linear form (form III). Incubation of pBR322 (19.3 µM/bp) at pH 7.5 and 37 °C with AuNP1-6 (45 µM) with and without added Zn(II) provided a reactivity picture that totally reversed what we observed in the cleavage of BNP: the worst catalyst (AuNP4-Zn(II)) became the best, while the best (AuNP1-Zn(II)) became the worst. Notably, AuNP4 and AuNP5 were significantly active even in the absence of Zn(II). AuNP6 featuring the same peptide of AuNP4 but lacking the Zn(II) chelating moiety was not active. The time course of the cleavage of plasmid pBR322 by AuNP4-Zn(II) is reported in Figure 3.
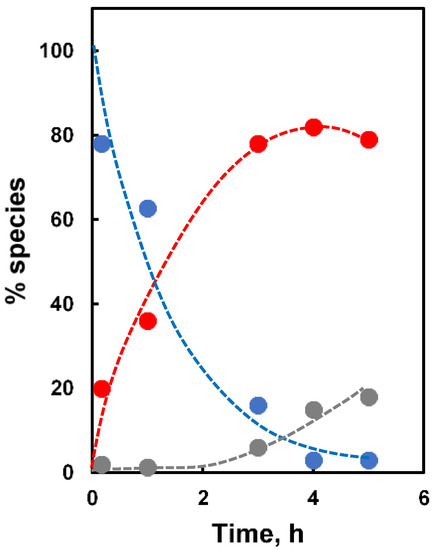
Figure 3.
Time course of the cleavage of plasmid pBR322 by AuNP4-Zn(II). Color codes: blue, supercoiled plasmid (form I); red, nicked circular (form II); gray, linear (form III).
The striking reversal of the activity between AuNP1-Zn(II) (the best catalysts for BNP hydrolysis) and AuNP4-Zn(II) (the best catalyst for the cleavage of the plasmid) suggested a difference in the catalytic mechanism. The collected data provided evidence that the best nanozyme for the DNA cleavage (AuNP4-Zn(II)) operates as a mononuclear catalyst and that in its catalytic site the guanidinium of Arg and the hydroxyl of a Ser play relevant roles by stabilizing the transition state and acting as a nucleophile, respectively.
5. Conclusions
The experimental evidence for the cleavage of BNP and plasmid DNA points to quite different mechanisms. In the case of BNP, two metal ions cooperate by coordinating the substrate phosphate and delivering a nucleophilic species (Figure 4A). The mechanism for DNA, however, still requires the phosphate diester to be coordinated to the metal ion, but the second metal ion is replaced by a positively charged arginine that assists with transition state stabilization and depresses the pKa of the hydroxyl group of serine (Figure 4B). The role played by the metal ion and the guanidinium is consistent with the observation that in phosphate-cleaving enzymes, basic groups of amino acids are not typically found to coordinate directly the reactive phosphate [10].
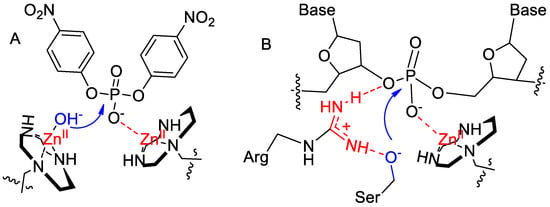
Figure 4.
Proposed mechanism of cleavage of BNP (A) and plasmid DNA (B) by gold nanozymes.
AuNP4-Zn(II) represents the best monometallic Zn(II)-based catalyst reported so far for the cleavage of DNA.
References
- Wolfenden, R. Benchmark reaction rates, the stability of biological molecules in water, and the evolution of catalytic power in enzymes. Annu. Rev. Biochem. 2011, 80, 645–667. [Google Scholar] [CrossRef] [PubMed]
- Yang, W. Nucleases: Diversity of structure, function and mechanism. Q. Rev. Biophys. 2011, 44, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Mancin, F.; Scrimin, P.; Tecilla, P. Progress in artificial metallonucleases. Chem. Commun. 2012, 48, 5545–5559. [Google Scholar] [CrossRef] [PubMed]
- Diez-Castellnou, M.; Martinez, A.; Mancin, F. Phosphate ester hydrolysis: The path from mechanistic investigation to the realization of artificial enzymes. Adv. Phys. Org. Chem. 2017, 51, 129–186. [Google Scholar]
- Czescik, J.; Zamolo, S.; Darbre, T.; Mancin, F.; Scrimin, P. Factors Influencing the Activity of Nanozymes in the Cleavage of an RNA Model Substrate. Molecules 2019, 24, 2814. [Google Scholar] [CrossRef] [PubMed]
- Czescik, J.; Zamolo, S.; Darbre, T.; Rigo, R.; Sissi, C.; Pecina, A.; Riccardi, L.; De Vivo, M.; Mancin, F.; Scrimin, P. A Gold Nanoparticle Nanonuclease Relying on a Zn (II) Mononuclear Complex. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Yatsimirsky, A. Metal ion catalysis in acyl and phosphoryl transfer: Transition states as ligands. Coordin. Chem. Rev. 2005, 249, 1997–2011. [Google Scholar] [CrossRef]
- Gabrielli, L.; Prins, L.J.; Rastrelli, F.; Mancin, F.; Scrimin, P. Hydrolytic Nanozymes. Eur. J. Org. Chem. 2020, 5044–5505. [Google Scholar] [CrossRef]
- Manea, F.; Houillon, F.B.; Pasquato, L.; Scrimin, P. Nanozymes: Gold-Nanoparticle-Based Transphosphorylation Catalysts. Angew. Chem. Int. Ed. Engl. 2004, 43, 6165–6169. [Google Scholar] [CrossRef] [PubMed]
- Genna, V.; Colombo, M.; De Vivo, M.; Marcia, M. Second-shell basic residues expand the two-metal-ion architecture of DNA and RNA processing enzymes. Structure 2018, 26, 40–50.e2. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).