Synthesis and Characterization of Graphene-Oxide Reinforced Copper Matrix Composite †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cu/GO Composite
2.2. Characterization
3. Results and Discussion
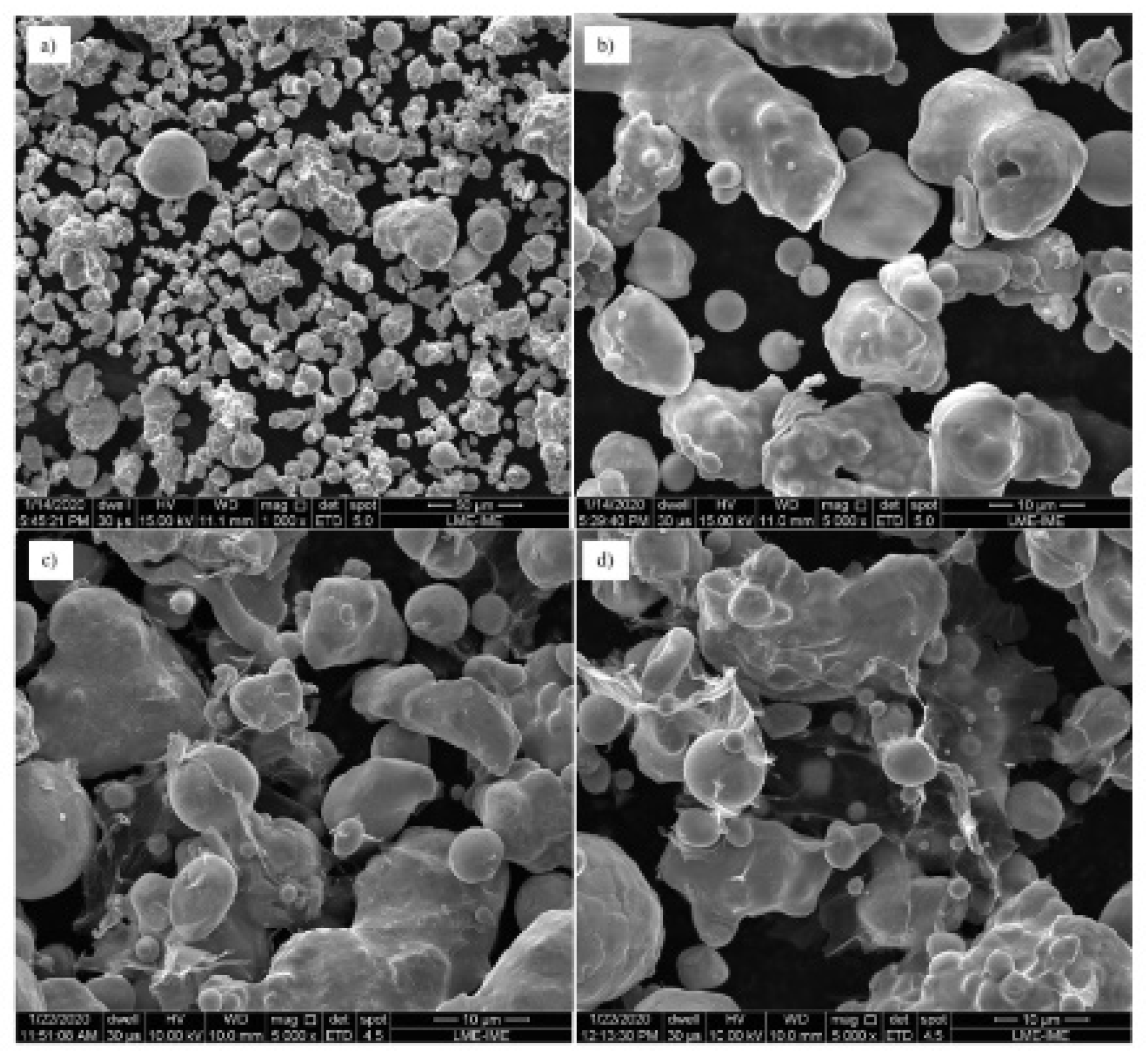
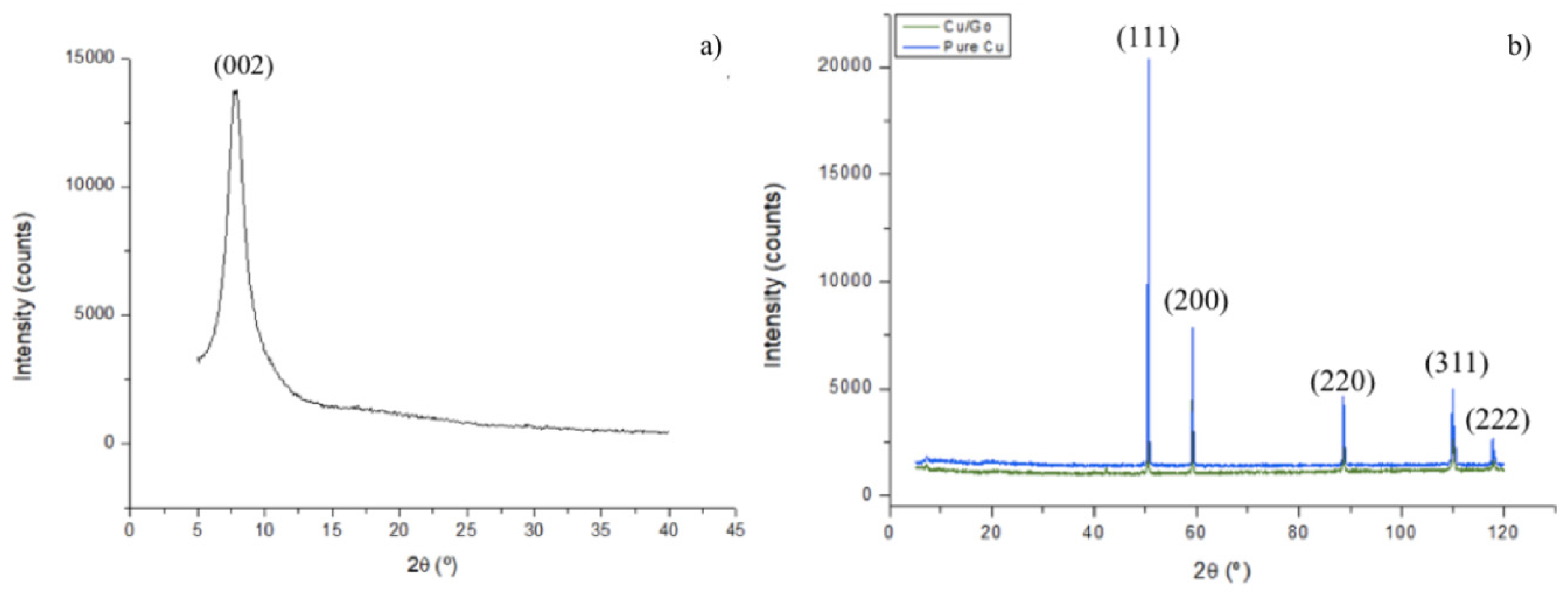
3.1. Powders and GO Morphology and Microstructural Analysis
3.2. Spectroscopy
3.2.1. Raman Analysis
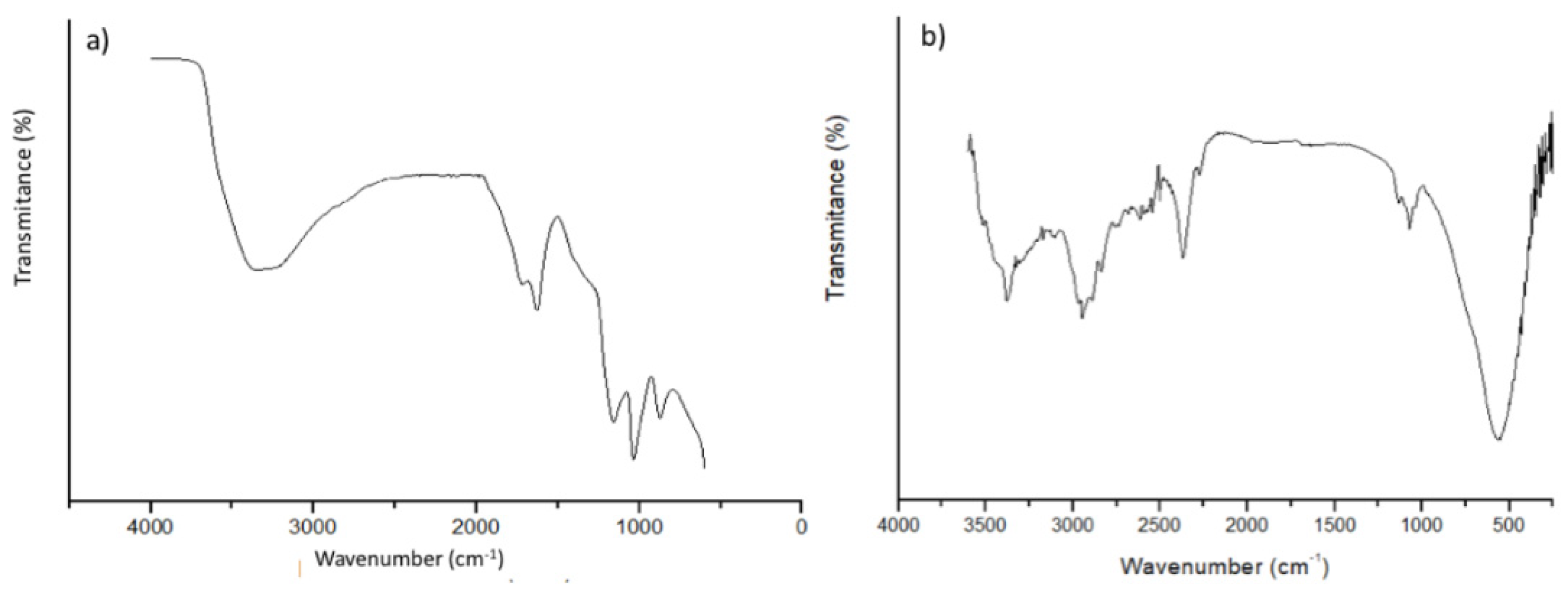
3.2.2. Fourier Transform Infrared Spectroscopy
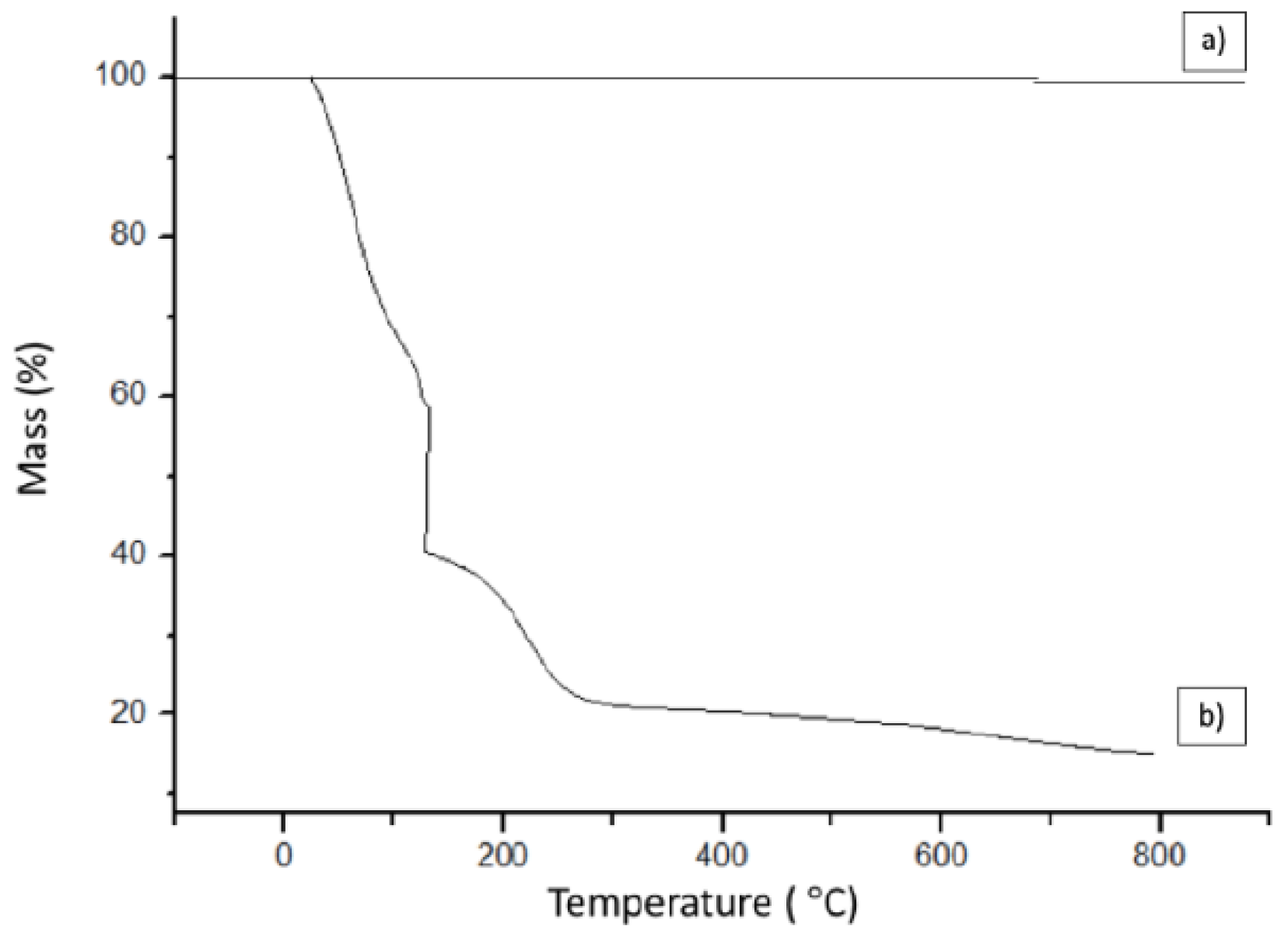
3.3. Thermogravimetric Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sousa, T.G.; Moura, I.A.B.; Filho, F.C.G.; Monteiro, S.N.; Brandão, L.P. Combining severe plastic deformation and precipitation to enhance mechanical strength and electrical conductivity of Cu–0.65Cr–0.08Zr alloy. J. Mater. Res. Technol. 2020, 9, 5953–5961. [Google Scholar] [CrossRef]
- Meng, A.; Nie, J.; Wei, K.; Kang, H.; Liu, Z.; Zhao, Y. Optimization of strength, ductility and electrical conductivity of a Cu–Cr–Zr alloy by cold rolling and aging treatment. Vacuum 2019, 167, 329–335. [Google Scholar] [CrossRef]
- Wang, J.; Guo, L.; Lin, W.; Chen, J.; Zhang, S.; Chen, S.; Zhen, T.; Zhang, Y. The effects of graphene content on the corrosion resistance, and electrical, termal and mechanical properties of graphene/copper composites. New Carbon Mater. 2019, 787, 786–793. [Google Scholar]
- Moghanian, A.; Sharifianjazi, F.; Abachi, P.; Sadeghi, E.; Jafarikhorami, H.; Sedghi, A. Production and properties of Cu/TiO2 nano-composites. J. Alloys Compd. 2017, 698, 518–524. [Google Scholar] [CrossRef]
- Selvakumar, V.; Muruganandam, S.; Senthilkumar, N. Evaluation of mechanical and tribological behavior of Al-4%Cu-x%SiC composites prepared through poder metallurgy technique. Trans. Indian Inst. Met. 2017, 70, 1305–1315. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based material: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Singh, M.; Yadav, A.; Kumar, S.; Agarwal, P. Annealing induced electrical conduction and band gap variation in thermally reduced graphene oxide films with different sp2/sp3 fraction. Appl. Surf. Sci. 2015, 326, 236–242. [Google Scholar] [CrossRef]
- Boostani, A.F.; Tahamtan, S.; Jiang, Z.Y.; Wei, D.; Yazdani, S.; Khosroshahi, R.A.; Mousavian, R.T.; Xu, J.; Zhang, X.; Gong, D. Enhanced tensile properties of aluminium matrix composites reinforced with graphene encapsulated SiC nanoparticles. Compos. Part A Appl. Sci. Manuf. 2015, 68, 155–163. [Google Scholar] [CrossRef]
- Baladin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior termal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Matter 2007, 6, 183. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.; Dubonos, S.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Alemany, L.B.; CI, L.; Ajayan, P.M. New insights into the structure and reduction of graphite oxide. Nat. Chem. 2009, 1, 403–408. [Google Scholar] [CrossRef]
- Jagannadham, K. Thermal conductivity of copper-graphene composite films synthesized by eletrochemical deposition with exfoliated graphene platelets Metall. Mater. Trans. 2011, 43, 316–324. [Google Scholar] [CrossRef]
- Hwang, J.; Yoon, T.; Jin, S.H.; Lee, J.; Kim, T.S.; Hong, S.H.; Jeon, S. Enhancedmechanical properties of graphene/copper nanocomposites using a molecular-level mixing process. Adv. Mater. 2013, 25, 6724–6729. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Ozga, P.; Maziarz, W.; Pstruś, J.; Kania, B.; Bobrowski, P.; Stolarska, J. Microstructure and properties of bulk copper matrix composites strengthened with various kinds of graphene nanoplatelets. Mater. Sci. Eng. A 2015, 628, 124–134. [Google Scholar] [CrossRef]
- Tian, Y.; Cheung, H.C.; Zheng, R.; Ma, Q.; Chen, Y.; Delepine-Gilon, N.; Yu, J. Elemental analysis of powders with surface-assisted thin film laser-induced breakdown spectroscopy. Spectrochim. Acta Part B 2016, 124, 16–24. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R. E Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1957, 208, 1937–1957. [Google Scholar] [CrossRef]
- Rourke, J.P.; Pandey, P.A.; Moore, J.J.; Bates, M.; Kinloch, I.A.; Young, R.J.; Wilson, N.R. The Real Graphene Oxide Revealed: Stripping the Oxidative Debris from the Graphene-like Sheets. Angew. Chem. Int. 2011, 50, 3173–3177. [Google Scholar] [CrossRef]
- Faria, G.S.; Lima, A.M.; Pinheiro, W.A.; Ribeiro, A.A.; Brandão, L.P.M. Compósitos cobre-grafeno produzidos por metalurgia do pó com compactação a frio. In Proceedings of the 72nd ABM Annual Congress, São Paulo, Brazil, 2–6 October 2017; Volume 72, pp. 2553–2560. [Google Scholar]
- Swain, K.A.; Bahadur, D. Enhanced Stability of Reduced Graphene Oxide Colloid Using Cross-Linking Polymers. J. Phys. Chem. C. 2014, 118, 9450–9457. [Google Scholar] [CrossRef]
- Yue, H.; Yao, L.; Gao, X.; Zhang, S.; Guo, E.; Zhang, H.; Lin, X.; Wang, B. Effect of ball-milling and graphene contents on themechanical properties and fracturemechanisms of graphene nanosheets reinforced copper matrix composites. J. Alloys Compd. 2017, 691, 755–762. [Google Scholar] [CrossRef]
- Gao, X.; Yue, H.; Guo, E.; Zhang, H.; Lin, X.; Yao, L.; Wang, B. Mechanical properties and thermal conductivity of graphene reinforced copper matrix composites. Powder Technol. 2016, 301, 601–607. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Pruna, A.; Pullini, D.; Busquets, D. Influence of synthesis conditions on properties of green-reduced graphene oxide. J. Nanoparticle Res. 2013, 15, 1605. [Google Scholar] [CrossRef]
- COROS, M.; Pogacean, F.; Turza, A.; Dan, M.; Berghian-Grosan, C.; Pana, I.-O.; Pruneanu, S. Green synthesis, characterization and potential application of reduced graphene oxide. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 119, 113971. [Google Scholar] [CrossRef]
- Ghadim, E.E.; Rashidi, N.; Kimiagar, S.; Akhavan, O.; Manouchehri, F.; Ghaderi, E. Pulsed laser irradiation for environment friendly reduction of graphene oxide suspensions. Appl. Surf. Sci. 2014, 301, 183–188. [Google Scholar] [CrossRef]
- Loryuenyong, V.; Totepvimarn, K.; Eimburanapravat, P.; Boonchompoo, W.; Buasri, A. Preparation and characterization of reduced graphene oxide sheets via water-based exfoliation and reduction methods. Ann. Mater. Sci. Eng. 2013, 352, 403–411. [Google Scholar] [CrossRef]
- Sarkar, C.; Dolui, S.K. Synthesis of copper oxide/reduced graphene oxide nanocomposite and its enhanced catalytic activity towards reduction of 4- nitrophenol. RSC Adv. 2015, 5, 60–72. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Zhang, F.; Wang, Y.; Chen, J. In Situ synthesis of three-dimensional graphene skeleton copper azide with tunable sensitivity performance. Mater. Lett. 2020, 279, 128466. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, I.A.d.B.; Sousa, T.G.d.; Lima, A.M.; Oliveira da Silva, W.; Brandao, L.P. Synthesis and Characterization of Graphene-Oxide Reinforced Copper Matrix Composite. Mater. Proc. 2021, 4, 72. https://doi.org/10.3390/IOCN2020-08000
Moura IAdB, Sousa TGd, Lima AM, Oliveira da Silva W, Brandao LP. Synthesis and Characterization of Graphene-Oxide Reinforced Copper Matrix Composite. Materials Proceedings. 2021; 4(1):72. https://doi.org/10.3390/IOCN2020-08000
Chicago/Turabian StyleMoura, Isaque Alan de Brito, Talita Gama de Sousa, Andreza Menezes Lima, Wesley Oliveira da Silva, and Luiz Paulo Brandao. 2021. "Synthesis and Characterization of Graphene-Oxide Reinforced Copper Matrix Composite" Materials Proceedings 4, no. 1: 72. https://doi.org/10.3390/IOCN2020-08000
APA StyleMoura, I. A. d. B., Sousa, T. G. d., Lima, A. M., Oliveira da Silva, W., & Brandao, L. P. (2021). Synthesis and Characterization of Graphene-Oxide Reinforced Copper Matrix Composite. Materials Proceedings, 4(1), 72. https://doi.org/10.3390/IOCN2020-08000







