Characterization of Chitosan Nanocapsules as a Biocompatible Polymeric System †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
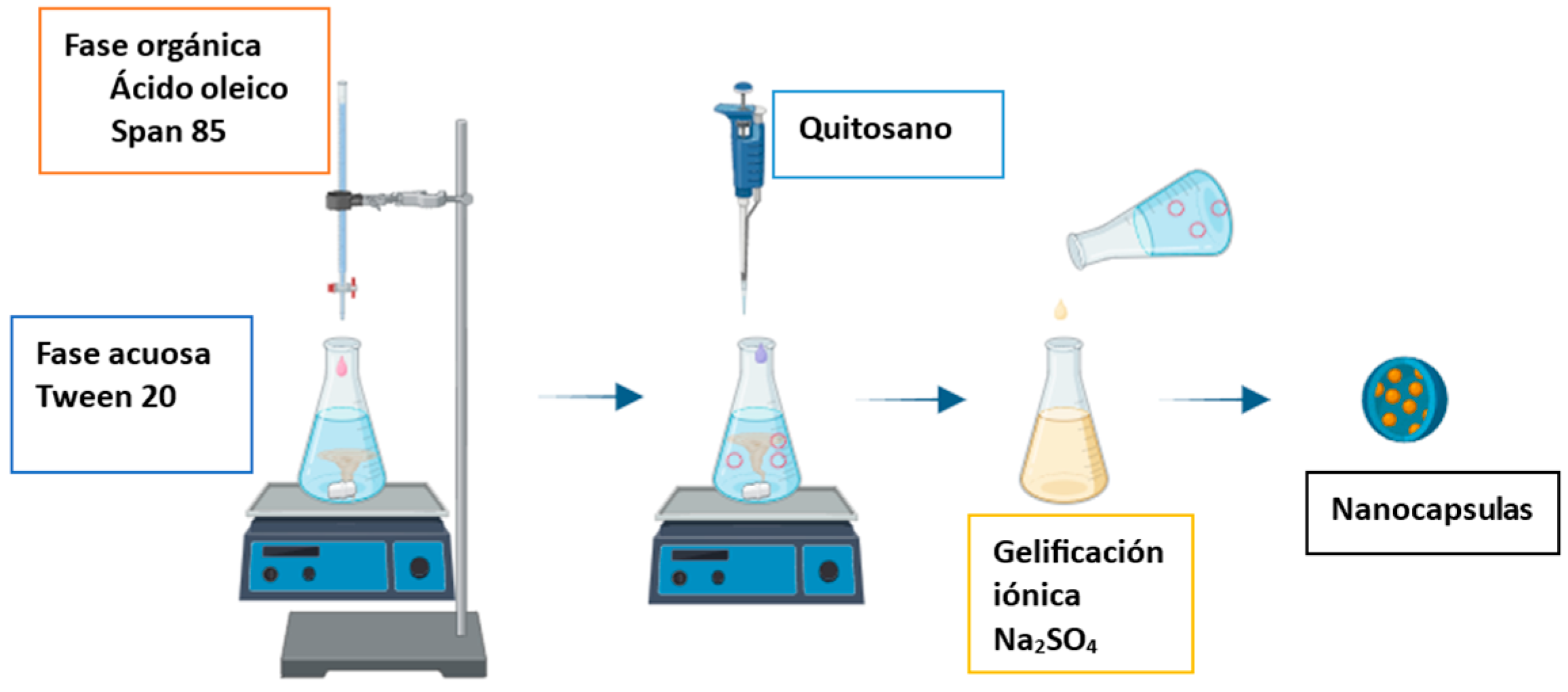
2.2. Synthesis of Nanocapsules
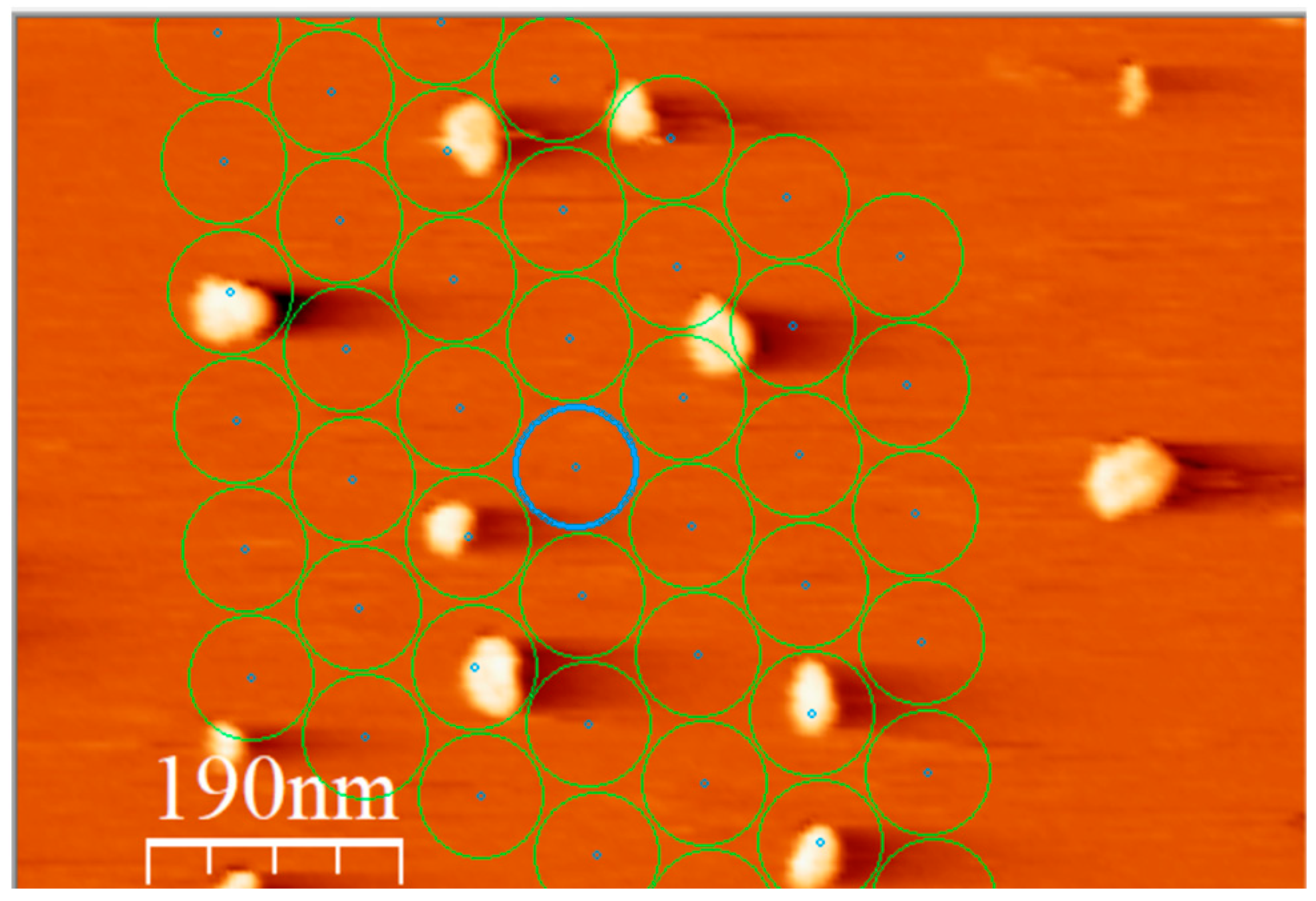
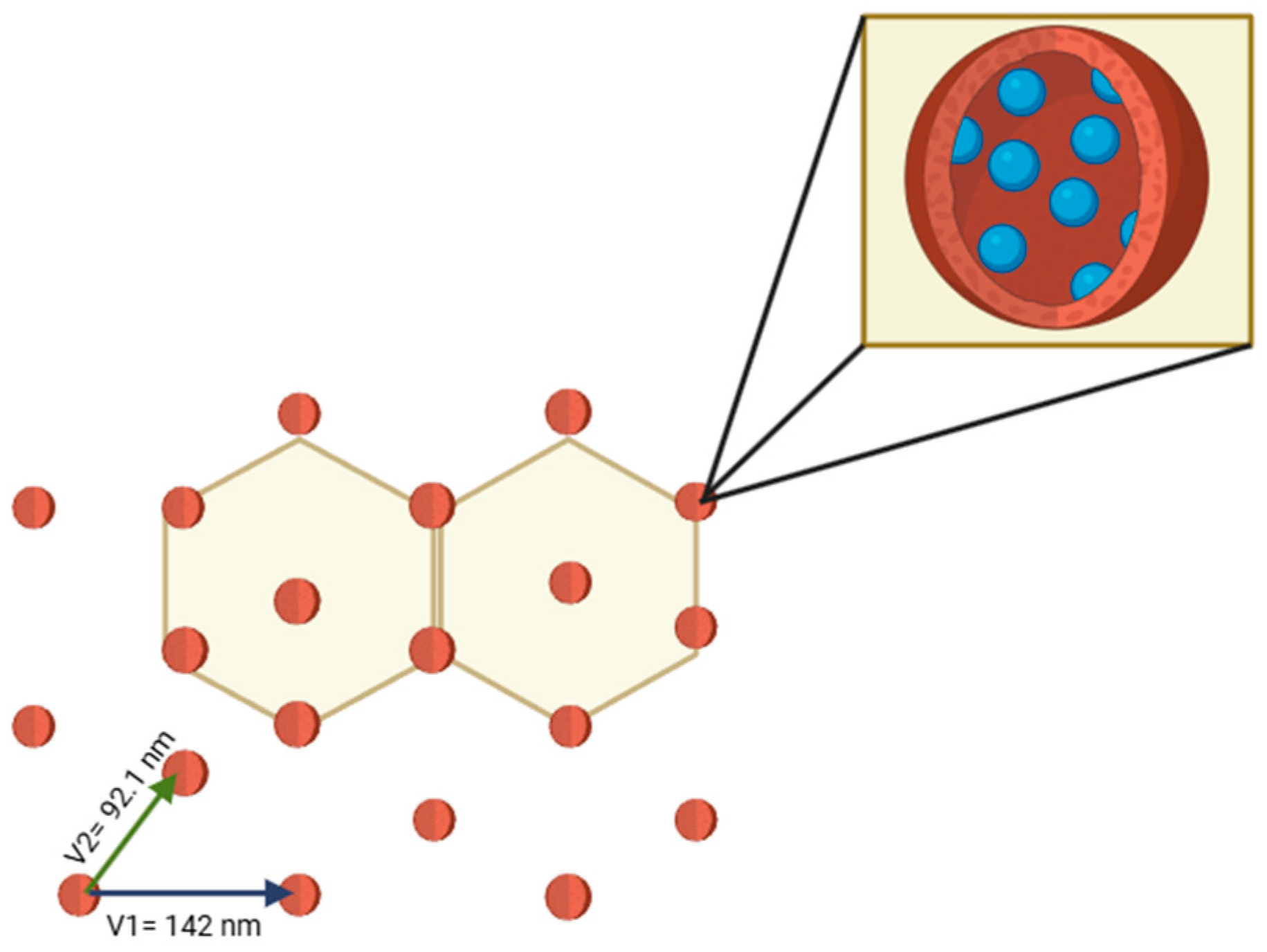
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhushan, B. Springer Nanotechnology Manual, 4th ed.; Springer: Berlin, Germeny, 2017. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional nanocarriers for the delivery of drugs and biologically active substances. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Applications of nanobiotechnology in clinical diagnostics. Clin. Chem. 2007, 53, 2002–2009. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances in chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.P.; Neufeld, R.J.; Ribeiro, A.J.; Veiga, F.; Nanoencapsulation, I. Methods for the preparation of drug-loaded polymeric nanoparticles. Nanomedicine 2006, 2, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Maity, S.; Mandal, S.; Chakraborti, A.S.; Prajapati, A.K.; Kundu, P.P. preparation, characterization and in vivo evaluation of pH sensitive, safe quercetin-succinylated chitosan-alginate core-shell-coronana noparticule for diabetes treatment. Carbohydr. Polym. 2014, 112, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 13705. [Google Scholar] [CrossRef] [PubMed]
- Jambhulkar, S.; Ravichandran, D.; Zhu, Y.; Thippanna, V.; Ramanathan, A.; Patil, D.; Fonseca, N.; Thummalapalli, S.V.; Sundaravadivelan, B.; Sun, A.; et al. Nanoparticle Assembly: From Self-Organization to Controlled Micropatterning for Enhanced Functionalities. Small 2024, 20, 2306394. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, R.E.R.; Urbieta, A.C.; Hernández, F.E.V.; Cruz, G.S.; Ochoa, J.J.; Ramírez, A.J.B.; Santiago, I.A.D. Characterization of Chitosan Nanocapsules as a Biocompatible Polymeric System. Mater. Proc. 2025, 28, 5. https://doi.org/10.3390/materproc2025028005
Cruz RER, Urbieta AC, Hernández FEV, Cruz GS, Ochoa JJ, Ramírez AJB, Santiago IAD. Characterization of Chitosan Nanocapsules as a Biocompatible Polymeric System. Materials Proceedings. 2025; 28(1):5. https://doi.org/10.3390/materproc2025028005
Chicago/Turabian StyleCruz, Rodrigo Emmanuel Ruiz, Antonio Canseco Urbieta, Francisco Emanuel Velásquez Hernández, Gabriel Sánchez Cruz, Joel Jiménez Ochoa, Alfonso Jesús Bautista Ramírez, and Ivonne Arisbeth Díaz Santiago. 2025. "Characterization of Chitosan Nanocapsules as a Biocompatible Polymeric System" Materials Proceedings 28, no. 1: 5. https://doi.org/10.3390/materproc2025028005
APA StyleCruz, R. E. R., Urbieta, A. C., Hernández, F. E. V., Cruz, G. S., Ochoa, J. J., Ramírez, A. J. B., & Santiago, I. A. D. (2025). Characterization of Chitosan Nanocapsules as a Biocompatible Polymeric System. Materials Proceedings, 28(1), 5. https://doi.org/10.3390/materproc2025028005



