Structural, Optical, and Dielectric Behavior of MCr2O4 (M=Co, Cu, Ni) Spinel Chromites Prepared by Sol–Gel Route †
Abstract
1. Introduction
2. Experimental
2.1. Synthesis Procedure
2.2. Characterization Techniques
3. Results and Discussion
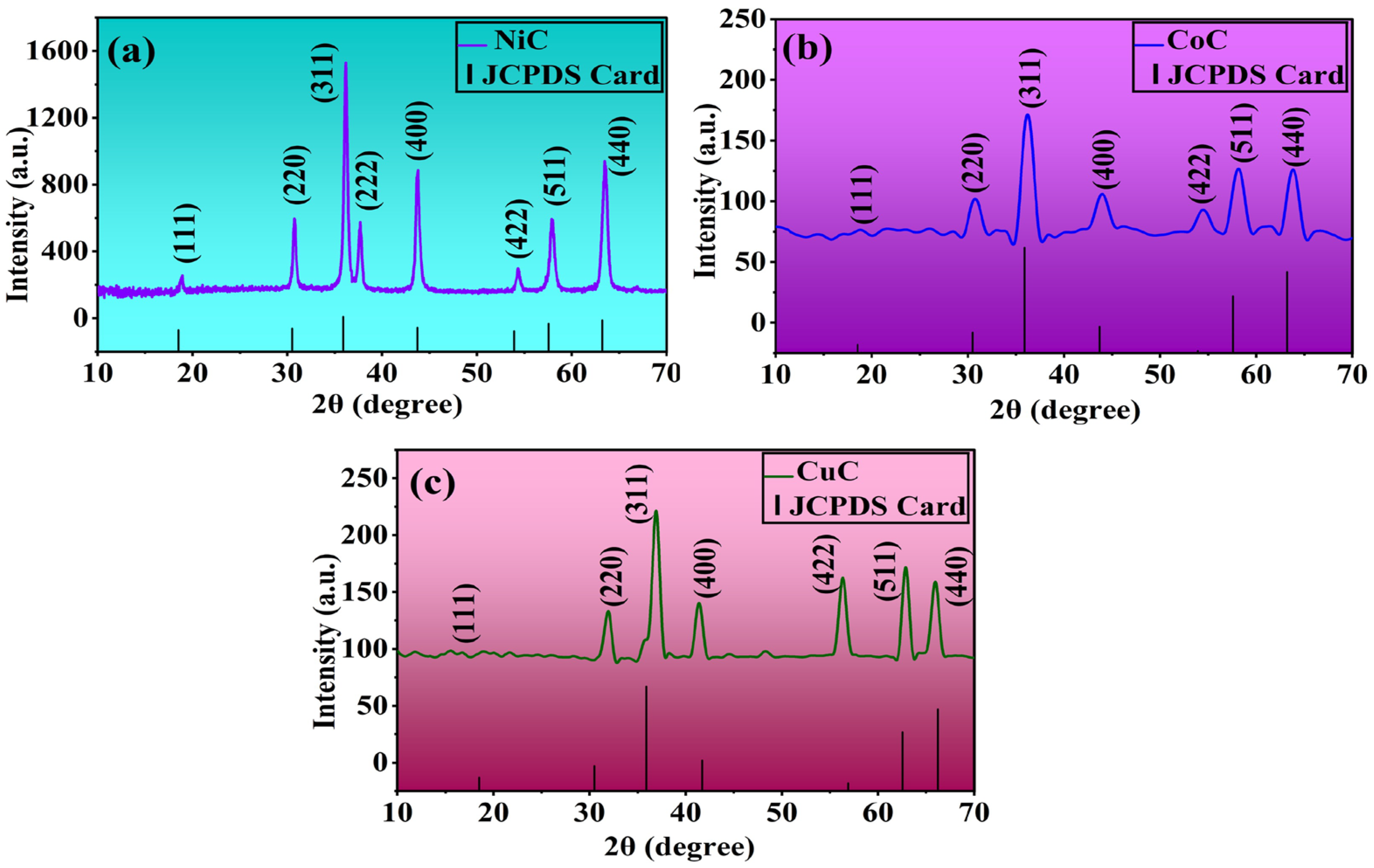
3.1. XRD Analysis
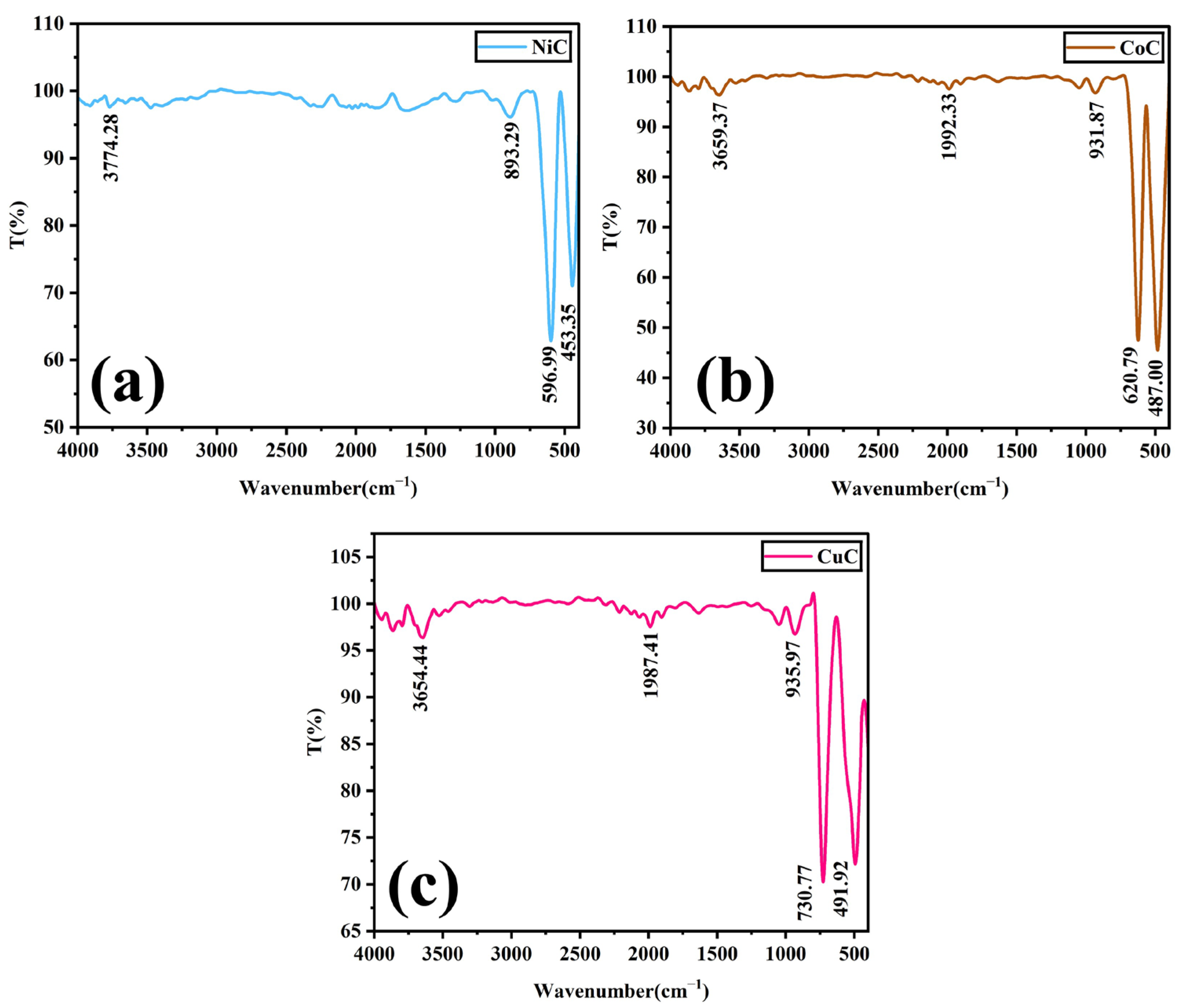
3.2. FTIR Analysis
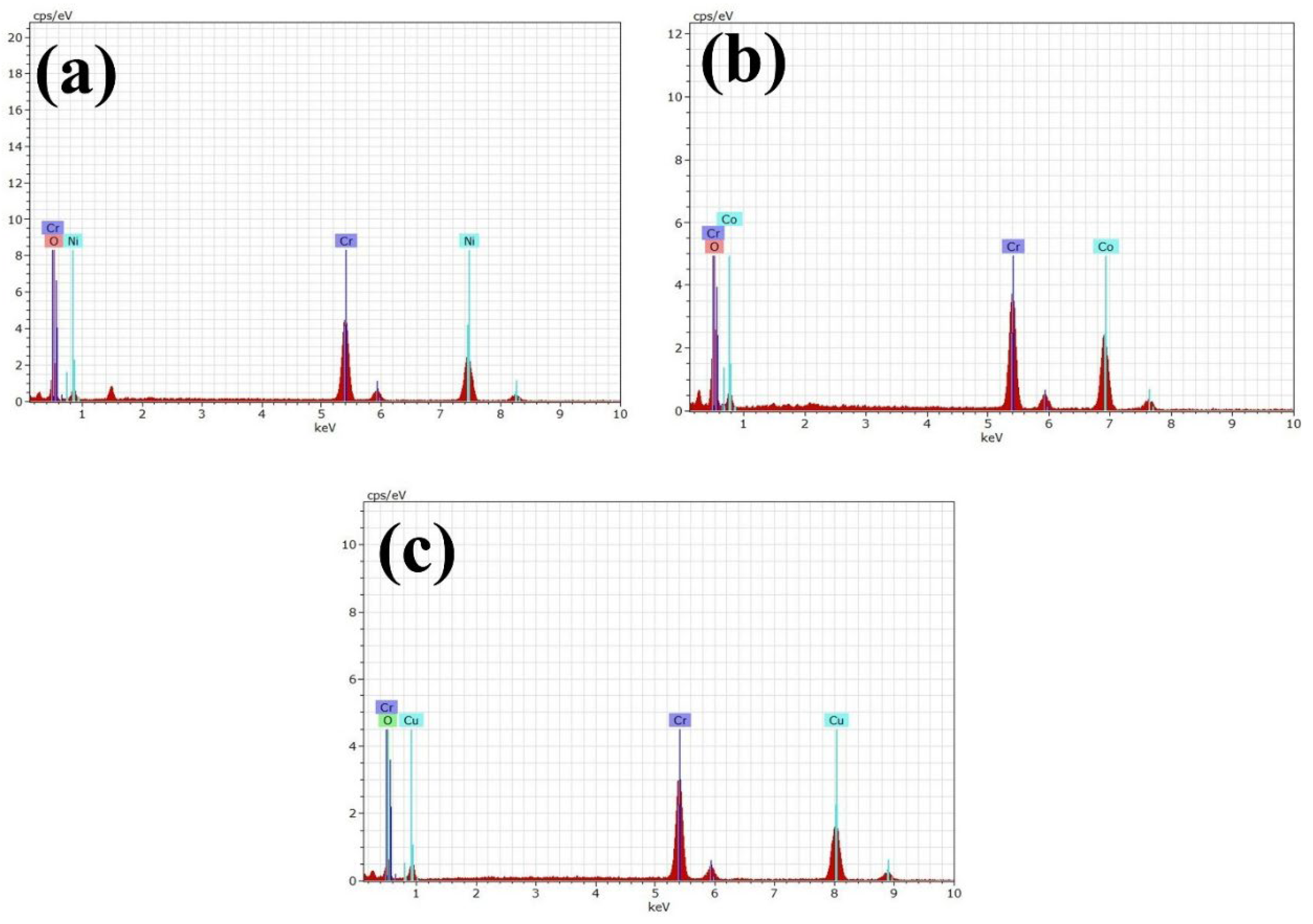
3.3. SEM Analysis
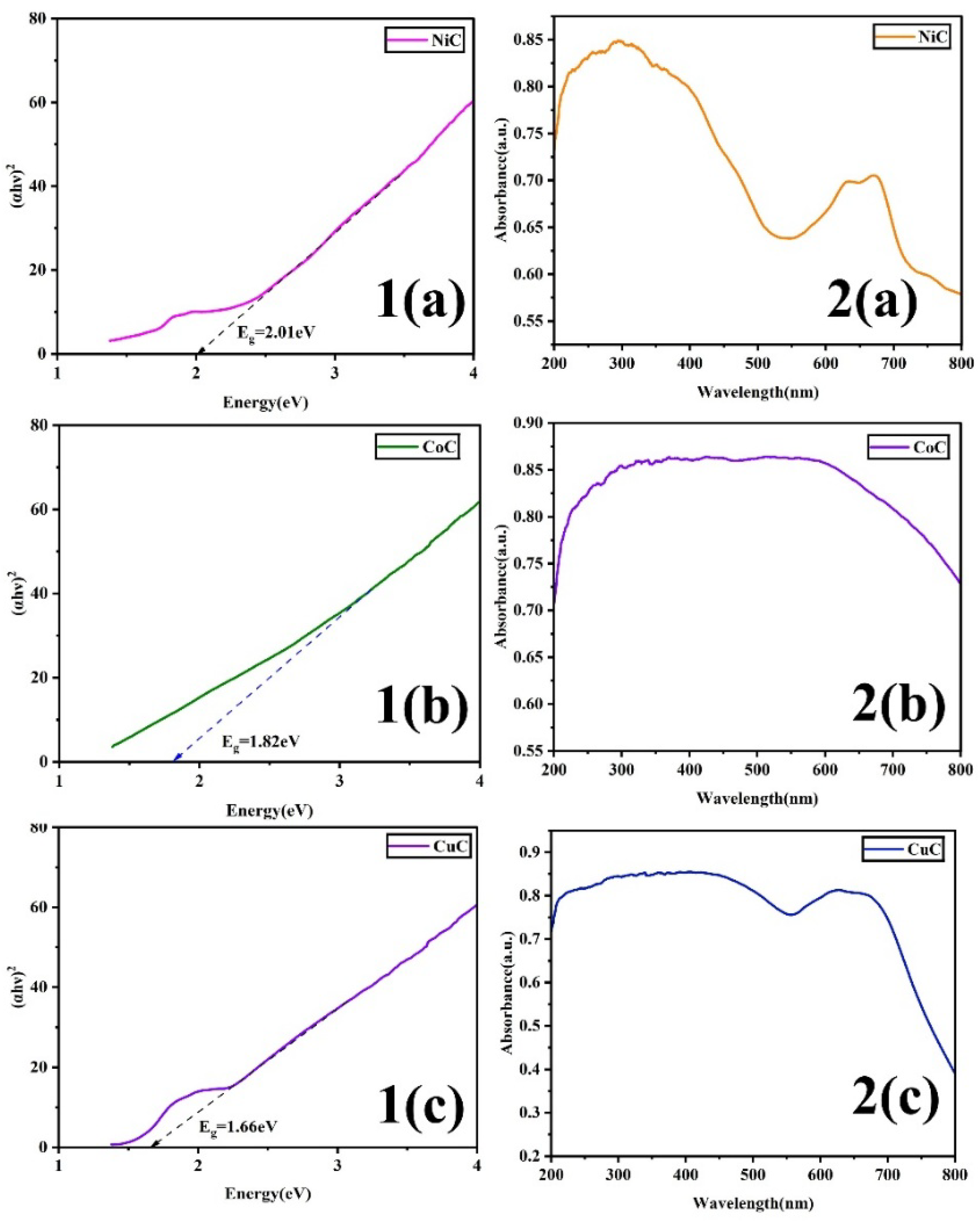
3.4. Optical Analysis
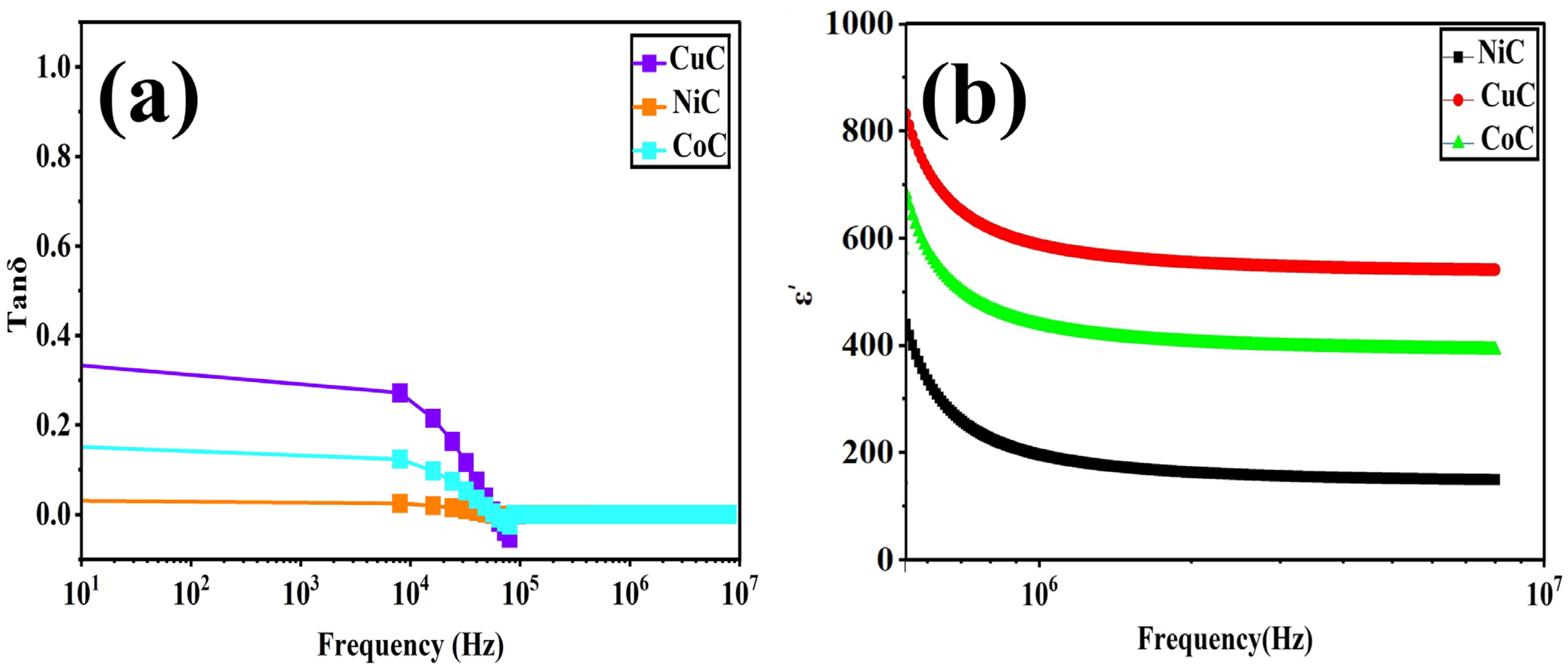
3.5. Dielectric Property Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anitha, G.; Pavithra, G.; Geetha, D. Tailoring ZnAl2O4 properties through divalent metal ions (Cu2+, Mg2+, & Mn2+) incorporation: A study on dielectric and photocatalytic enhancement. J. Mater. Sci. Mater. Electron. 2025, 36, 1–27. [Google Scholar] [CrossRef]
- Anandha Kumar, S.; Shahanas, T.; Harichandran, G. Synthesis and fabrication of tetragonal copper chromite nanospheres for usage as the cathode material in asymmetric supercapacitors. J. Energy Storage 2025, 106, 114839. [Google Scholar] [CrossRef]
- Mamidipalli, N.R.; Tiyyagura, P.; Punna Rao, S.; Kothamasu, S.B.; Pothu, R.; Boddula, R.; Al-Qahtani, N. Crystallite Size Effects on Electrical Properties of Nickel Chromite (NiCr2O4) Spinel Ceramics: A Study of Structural, Magnetic, and Dielectric Transitions. ChemEngineering 2024, 8, 100. [Google Scholar] [CrossRef]
- Ajith, A.; Wani, T.A.; Suresh, G.; Saravanan, N.; Masrour, R. Sol–Gel Synthesis and Characterizations of Spinel Nickel Chromite (NiCr2O4) Nanoparticles to Study their Photocatalytic, Antibacterial and Anticancer Activities. Nano 2024, 19, 2450050. [Google Scholar] [CrossRef]
- Kumar, K.V.; Bhavani, S.D. Influence of Calcination Temperature on Physicaland Optical Properties of Nickel Chromite Nanoparticles. Sci. Sinter. 2022, 54, 457–468. [Google Scholar] [CrossRef]
- Choudapur, V.H.; Alrashidi, K.A.; Sajjan, A.M.; Ayachit, N.H.; Adil, S.F.; Fernandes, B.J.; Angadi, V.J. Effect of Rare Earth (La3+) on the Structural, Microstructural, and Transport Properties of CoCr2O4 for Industrial Applications. Int. J. Self-Propagating High-Temp. Synth. 2024, 33, 258–265. [Google Scholar] [CrossRef]
- Bisht, N.; Rao, S.S.; Deshawal, S.; Khanna, P.K. Novel microwave synthesis of copper chromite nanoparticles. Inorg. Chem. Commun. 2021, 134, 109072. [Google Scholar] [CrossRef]
- Gurusamy, P.; Gnanasekar, A.; Deivasigamani, G. Investigation of Structural, Morphological, Optical, and Dielectric Properties of Magnesium Chromite (MgCr2O4) Spinel Oxide. Eng. Proc. 2025, 87, 109. [Google Scholar] [CrossRef]
- Priya, A.S.; Geetha, D.; Madhavan, J. Synthesis and Structural, Dielectric and Photocatalytic Properties of (Ti, La)-co-Doped Calcium Ferrite Ceramic Powders. Arab. J. Sci. Eng. 2022, 47, 7657–7667. [Google Scholar] [CrossRef]
- Garg, T.; Parmar, L.K.; Koser, A.A.; Kaurav, N.; Yadav, A. Impact of Zn-Ion Doping on the Structural and Optical Properties of Cobalt Chromite System. J. Mol. Eng. Mater. 2025, 13, 2–11. [Google Scholar] [CrossRef]

| Parameters | CoC | CuC | NiC |
|---|---|---|---|
| Crystallite size (D)nm | 6.73 | 9.86 | 10.73 |
| Micro Strain (ε) | 2.59 | 1.83 | 1.49 |
| Interplanar distance (d) Å | 2.02 | 1.99 | 2.14 |
| Lattice constant Å | 8.23 | 7.65 | 8.25 |
| Volume of the unit cell (a3) Å | 558.15 | 448.44 | 562.73 |
| Packing factor P | 3.21 | 4.95 | 4.99 |
| Density Dx (g/cm3) | 3.01 | 3.89 | 2.98 |
| Specific surface area S (cm2/g) | 2.95 | 1.56 | 1.87 |
| Stacking fault SF | 0.38 | 0.38 | 0.40 |
| Dislocation density (δ) (10−3/m2) | 0.022 | 0.010 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurusamy, P.; Gnanasekar, A.; Deivasigamani, G.; Mediano, J.L.A. Structural, Optical, and Dielectric Behavior of MCr2O4 (M=Co, Cu, Ni) Spinel Chromites Prepared by Sol–Gel Route. Mater. Proc. 2025, 25, 6. https://doi.org/10.3390/materproc2025025006
Gurusamy P, Gnanasekar A, Deivasigamani G, Mediano JLA. Structural, Optical, and Dielectric Behavior of MCr2O4 (M=Co, Cu, Ni) Spinel Chromites Prepared by Sol–Gel Route. Materials Proceedings. 2025; 25(1):6. https://doi.org/10.3390/materproc2025025006
Chicago/Turabian StyleGurusamy, Pavithra, Anitha Gnanasekar, Geetha Deivasigamani, and Jose Luis Arias Mediano. 2025. "Structural, Optical, and Dielectric Behavior of MCr2O4 (M=Co, Cu, Ni) Spinel Chromites Prepared by Sol–Gel Route" Materials Proceedings 25, no. 1: 6. https://doi.org/10.3390/materproc2025025006
APA StyleGurusamy, P., Gnanasekar, A., Deivasigamani, G., & Mediano, J. L. A. (2025). Structural, Optical, and Dielectric Behavior of MCr2O4 (M=Co, Cu, Ni) Spinel Chromites Prepared by Sol–Gel Route. Materials Proceedings, 25(1), 6. https://doi.org/10.3390/materproc2025025006





