Synthesis and Characterization of ZnSnO3/PVP Electrospun Composite Nanofibers †
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Synthesis of ZnSnO3 Nanoparticles
2.3. Preparation of ZnSnO3/PVP Electrospun Nanofibers
2.4. Electrospinning of Composite Nanofibers
2.5. Characterization Techniques
3. Results and Discussion
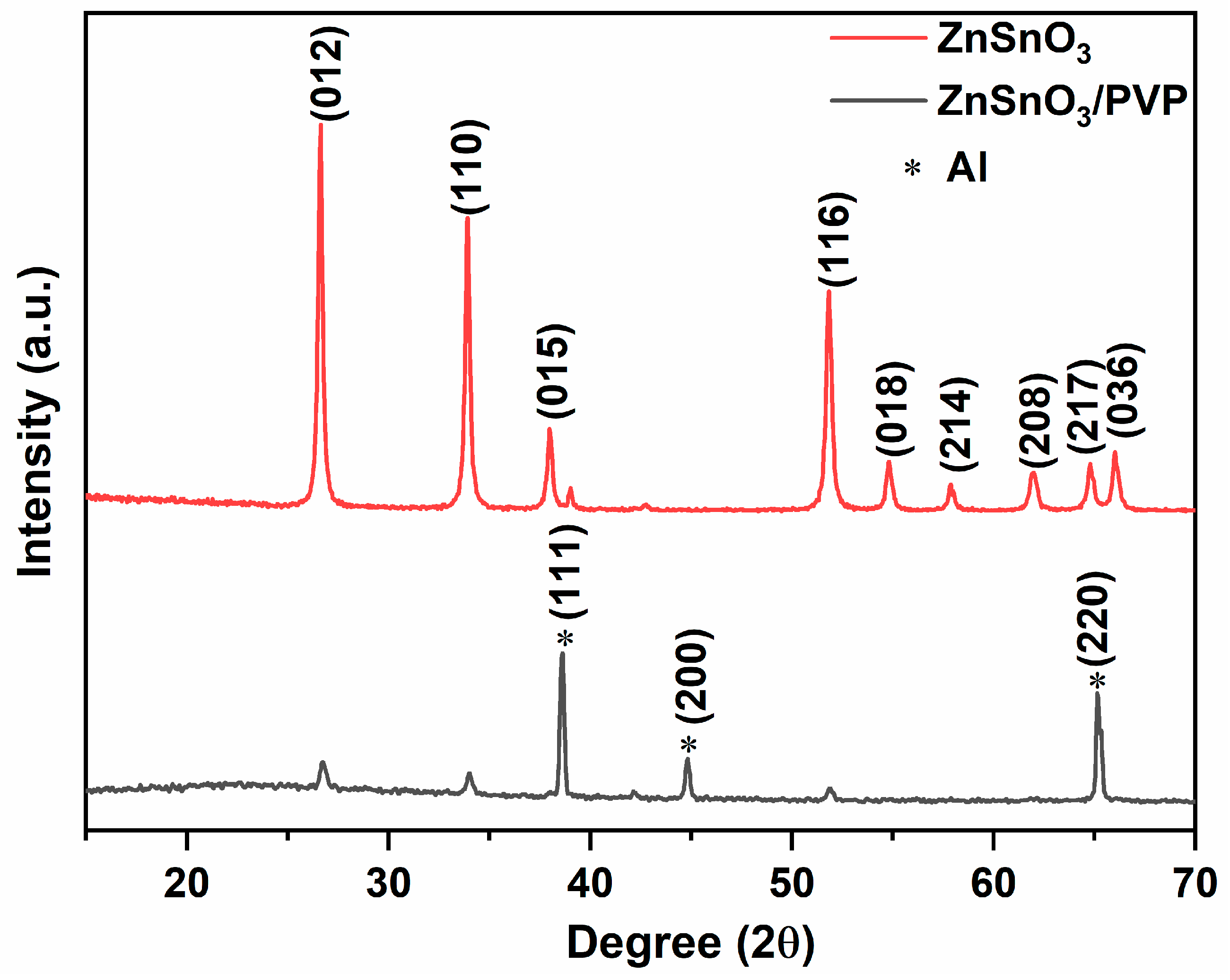
3.1. Structural Analysis
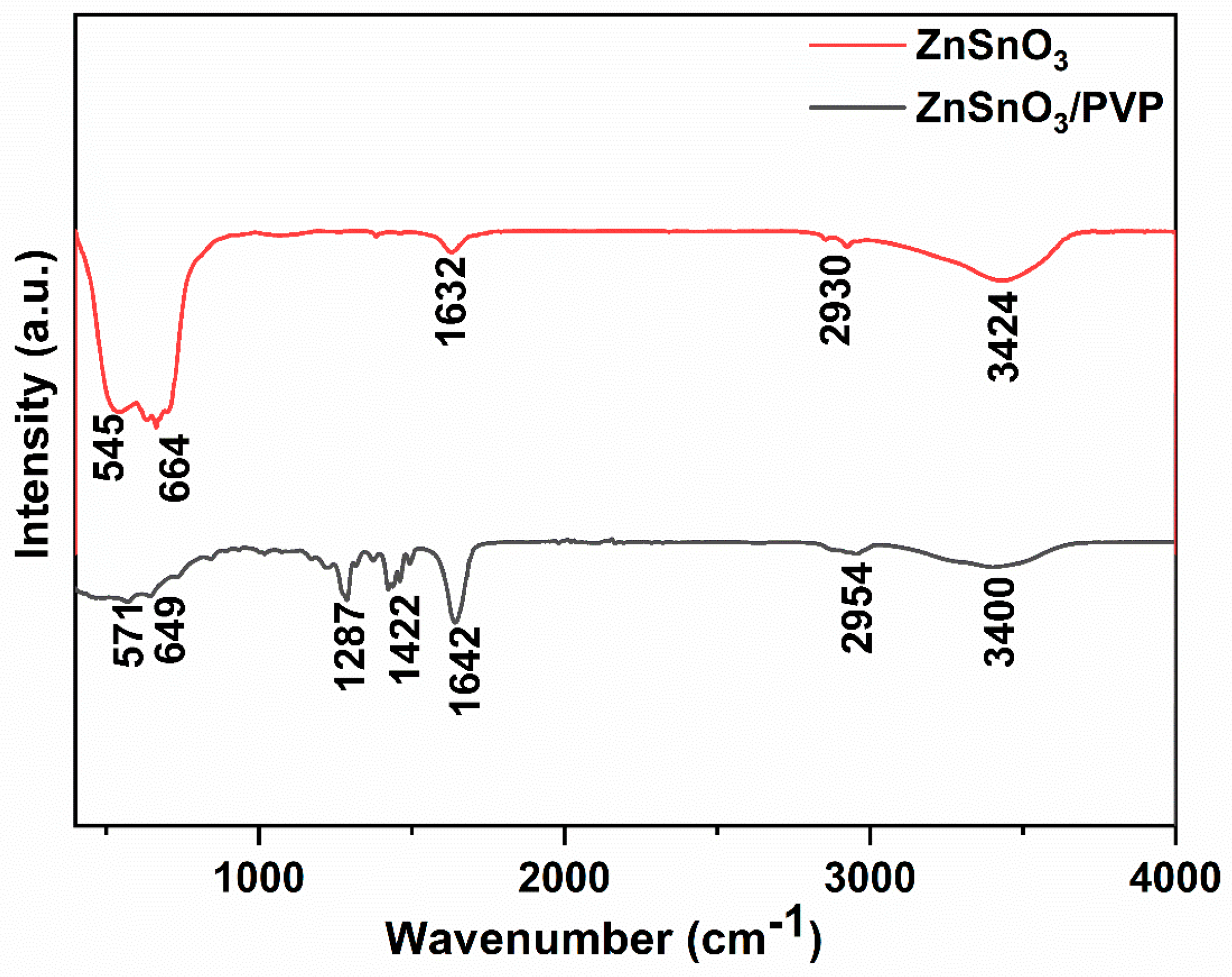
3.2. Infrared Spectra Analysis
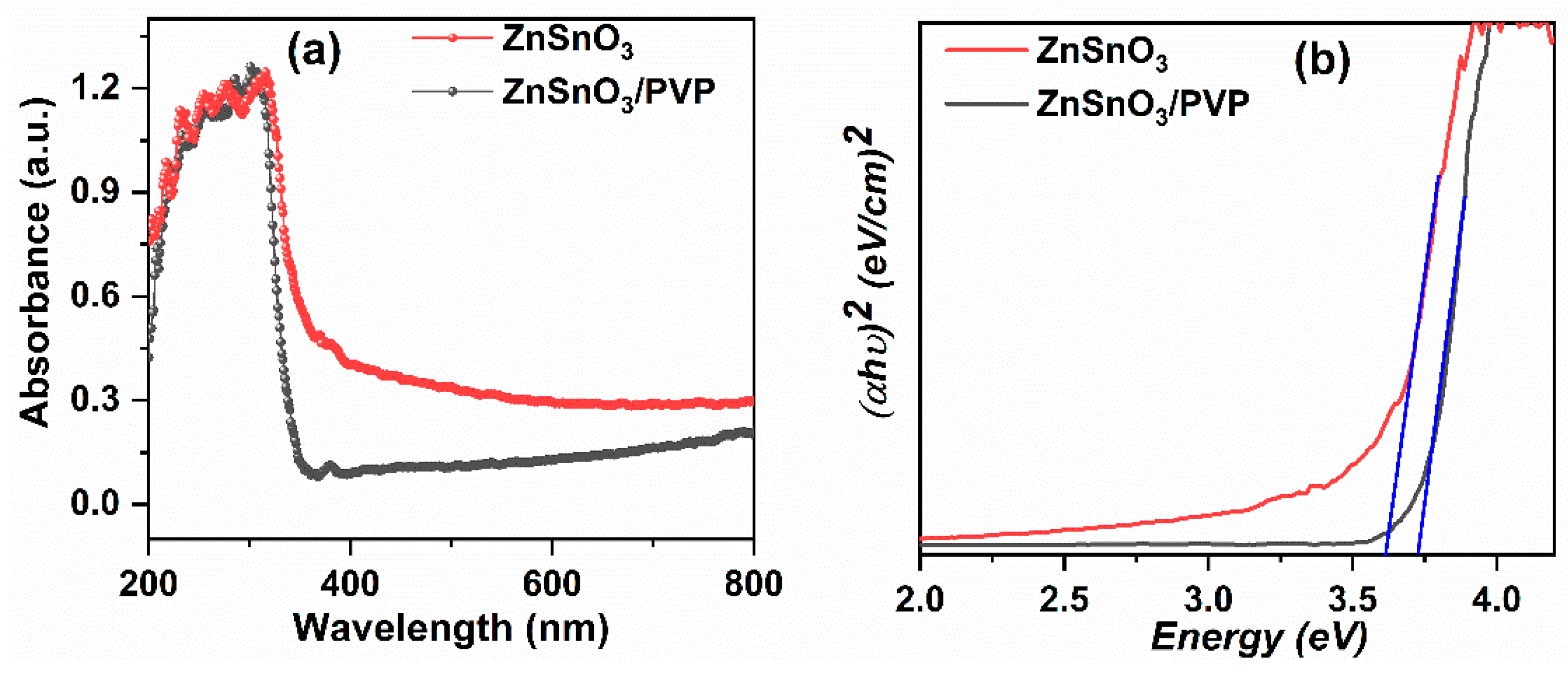
3.3. Optical Properties
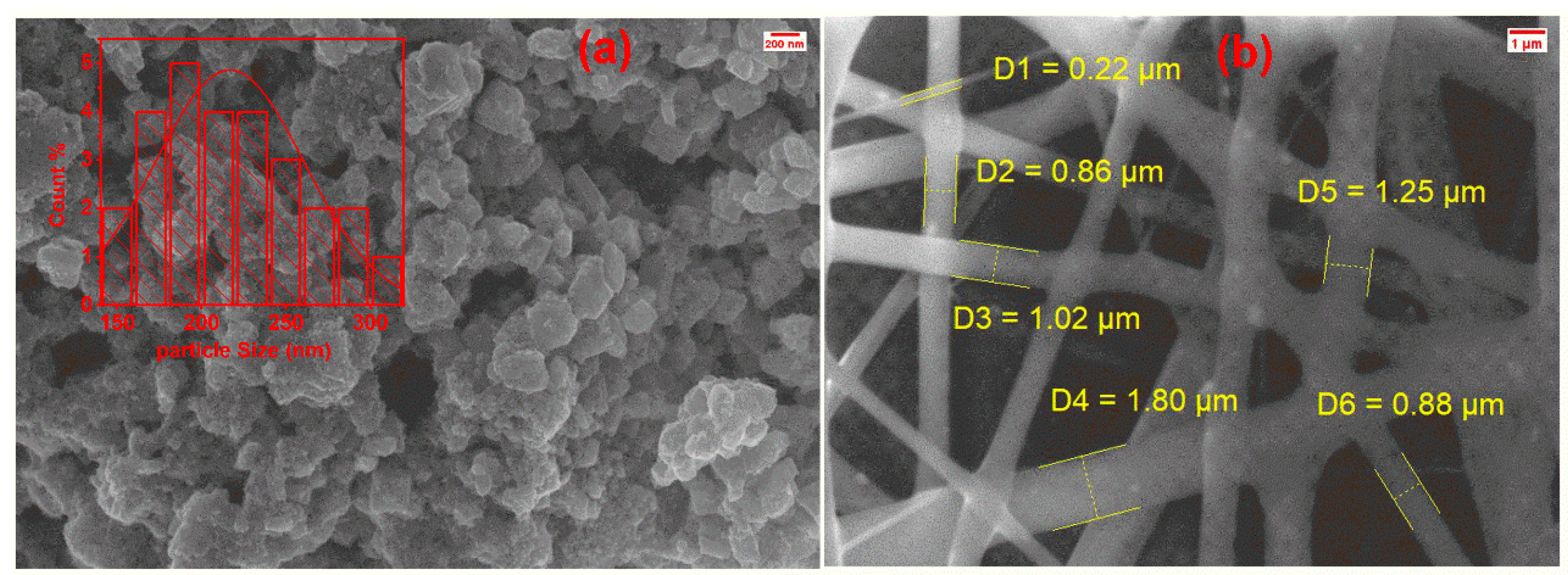
3.4. Morphology Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibrahim, D.M.; Gaber, A.A.; Reda, A.E.; Abdel Aziz, D.A.; Ajiba, N.A. Structural, optical, and dielectric properties of sol-gel derived perovskite ZnSnO3 nanomaterials. J. Sol-Gel Sci. Technol. 2024, 112, 703–714. [Google Scholar] [CrossRef]
- Mukherjee, D.; Datta, A.; Kons, C.; Hordagoda, M.; Witanachchi, S.; Mukherjee, P. Intrinsic anomalous ferroelectricity in vertically aligned LiNbO3-type ZnSnO3 hybrid nanoparticle-nanowire arrays. Appl. Phys. Lett. 2014, 105, 212903. [Google Scholar] [CrossRef]
- Anith, A.; Ponnusamy, V. Optical and electrochemical studies on single-phase ZnSnO3 nanostructures—A photosensitive approach. Surf. Interfaces 2024, 51, 104747. [Google Scholar]
- Rovisco, A.; dos Santos, A.; Cramer, T.; Martins, J.; Branquinho, R.; Aguas, H.; Fraboni, B.; Fortunato, E.; Martins, R.; Igreja, R.; et al. Piezoelectricity Enhancement of Nanogenerators Based on PDMS and ZnSnO3 Nanowires through Microstructuration. ACS Appl. Mater. Interfaces 2020, 12, 18421–18430. [Google Scholar] [CrossRef]
- George, S.D.B.; Nagarajan, K.; Ali, A.A.; Madamala, S.; Subramanian, D.; Kuppamuthu, S.; Aruckiasamy, J.J. Morphology-optimized ZnSnO3 nanopentagons as efficient electron transport layers for high-efficient perovskite solar cells. J. Solid. State Chem. 2025, 347, 125322. [Google Scholar] [CrossRef]
- Sim, C.K.; Majid, S.R.; Mahmood, N.Z. ZnSnO3/mesoporous biocarbon composite towards sustainable electrode material for energy storage device. Microchem. J. 2021, 164, 105968. [Google Scholar] [CrossRef]
- Dong, S.; Cui, L.; Zhang, W.; Xia, L.; Zhou, S.; Russell, C.K.; Fan, M.; Feng, J.; Sun, J. Double-shelled ZnSnO3 hollow cubes for efficient photocatalytic degradation of antibiotic wastewater. Chem. Eng. J. 2020, 384, 123279. [Google Scholar] [CrossRef]
- Choi, K.H.; Siddiqui, G.U.; Yang, B.-s.; Mustafa, M. Synthesis of ZnSnO3 nanocubes and thin film fabrication of (ZnSnO3/PMMA) composite through electrospray deposition. J. Mater. Sci. Mater. Electron. 2015, 26, 5690–5696. [Google Scholar] [CrossRef]
- Siddiqui, G.U.; Rehman, M.M.; Choi, K.H. Enhanced resistive switching in all-printed, hybrid and flexible memory device based on perovskite ZnSnO3 via PVOH polymer. Polymer 2016, 100, 102–110. [Google Scholar] [CrossRef]
- Prinscilla, J.; Vijayaraghavan, G.V.; Vilvanathaprabu, A.; Suriakarthick, R. Electrical and ferroelectric properties of novel PVDF/ZnSnO3 polymer nanocomposites for flexible energy storage application. J. Alloys Compd. 2025, 1037, 182303. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M. Poly(vinylpyrrolidone)—A Versatile Polymer for Biomedical and Beyond Medical Applications. Polym. Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Wang, P.; Lv, H.; Cao, X.; Liu, Y.; Yu, D.-G. Recent Progress of the Preparation and Application of Electrospun Porous Nanofibers. Polymers 2023, 15, 921. [Google Scholar] [CrossRef]
- Kim, O.S.; Kwon, J.B.; Kim, S.W.; Xu, B.; Seo, K.H.; Park, C.E.; Do, W.J.; Bae, J.H.; Kang, S.W. Effect of PVP-Capped ZnO Nanoparticles with Enhanced Charge Transport on the Performance of P3HT/PCBM Polymer Solar Cells. Polymers 2019, 11, 1818. [Google Scholar] [CrossRef]
- Geetha, K.; Sivasangari, D.; Kim, H.-S.; Murugadass, G.; Kathalingam, A. Electrospun nanofibrous ZnO/PVA/PVP composite films for efficient antimicrobial face masks. Ceram. Int. 2022, 48, 29197–29294. [Google Scholar] [CrossRef]
- Kavarthapu, V.S.; Graham, S.A.; Manchi, P.; Paranjape, M.V.; Yu, J.S. Electrospun ZnSnO3/PVDF-HFP Nanofibrous Triboelectric Films for Efficient Mechanical Energy Harvesting. Adv. Fiber Mater. 2023, 5, 1685–1698. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Zhuo, Z.; Dugnani, R.; Xue, W.; Zhou, Y.; Chen, Y.; Liu, H. Characterization of the damping and mechanical properties of a novel (ZnSnO3/PVDF)@PPy nanofibers/EP composite. RSC Adv. 2017, 7, 37130. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Wang, M.; Ma, S.; Wang, P.; Zhang, G.; Chen, W.; Jiao, H.; Liu, L.; Xu, X. Enhanced acetone sensor based on Au functionalized In-doped ZnSnO3 nanofibers synthesized by electrospinning method. J. Colloid. Interface Sci. 2019, 543, 285–299. [Google Scholar] [CrossRef]
- ul Haq, M.; Zhang, Z.; Chen, X.; Rahman, N.; Khan, S.; Khatoon, R.; Hassan, S.S.; Ye, Z.; Zhu, L. A two-step synthesis of microsphere-decorated fibers based on NiO/ZnSnO3 composites towards superior ethanol sensitivity performance. J. Alloys Compd. 2019, 777, 73–83. [Google Scholar] [CrossRef]
- Kovacheva, D.; Petrov, K. Preparation of crystalline ZnSnO from Li SnO by low-temperature ion exchange. Solid. State Ionics 1998, 109, 327–332. [Google Scholar] [CrossRef]
- Shujah, T.; Ikram, M.; Butt, A.R.; Hussain, S.G.; Shahzad, M.K.; Zafar, Q.; Ali, S. Growth of Zinc Oxide and Zinc Stannate Nanostructured Thin Films for Carbon Monoxide Sensing Application. Nanosci. Nanotechnol. Lett. 2019, 11, 1050–1059. [Google Scholar] [CrossRef]
- Klobes, B. Comment on Modification of Gas Sensitivity in ZnSnO3 Film from Carrier Behaviors: Derivation, Concentration, and Depletion: Rather SnO2 than ZnSnO3. J. Phys. Chem. C 2024, 128, 16824–16825. [Google Scholar] [CrossRef]
- Gaddam, V.; Kumar, R.R.; Parmar, M.; Yaddanapudi, G.R.K.; Nayak, M.M.; Rajanna, K. Morphology controlled synthesis of Al doped ZnO nanosheets on Al alloy substrate by low-temperature solution growth method. RSC Adv. 2015, 5, 13519–13524. [Google Scholar] [CrossRef]
- Atisme, T.B.; Yu, C.-Y.; Tseng, E.N.; Chen, Y.-C.; Hsu, P.-K.; Chen, S.Y. Interface Interactions in Conjugated Polymer Composite with Metal Oxide Nanoparticles. Nanomaterials 2019, 9, 1534. [Google Scholar] [CrossRef]
- Brahmi, C.; Benltifa, M.; Vaulot, C.; Michelin, L.; Dumur, F.; Airoudj, A.; Morlet-Savary, F.; Reveau, B.; Bousselmi, L.; Lalevée, J. New hybrid perovskites/polymer composites for the photodegradation of organic dyes. Eur. Polym. J. 2021, 157, 110641. [Google Scholar] [CrossRef]
- Indhumathi, R.; Priya, A.S.; Aepuru, R. Effect of calcination temperature on the structure, optical and dielectric properties of ZnSnO3 ceramics. Ceram. Int. 2025, 51, 55030–55055. [Google Scholar] [CrossRef]
- Sá, B.S.; Zito, C.A.; Perfecto, T.M.; Volanti, D.P. Porous ZnSnO3 nanocubes as a triethylamine sensor. Sens. Actuators B 2021, 338, 129869. [Google Scholar] [CrossRef]
- Bora, T.; Al-Hinai, M.H.; Al-Hinai, A.T.; Dutta, J. Phase Transformation of Metastable ZnSnO3 Upon Thermal Decomposition by In-Situ Temperature-Dependent Raman Spectroscopy. J. Am. Ceram. Soc. 2015, 98, 4044–4049. [Google Scholar] [CrossRef]
- Deniz, A.E.; Vural, H.A.; Ortaç, B.; Uyar, T. Gold nanoparticle/polymer nanofibrous composites by laser ablation and electrospinning. Mater. Lett. 2011, 65, 2941–2943. [Google Scholar] [CrossRef]
- Baganizi, D.R.; Nyairo, E.; Duncan, S.A.; Singh, S.R.; Vida, A. Interleukin-10 Conjugation to Carboxylated PVP-Coated Silver Nanoparticles for Improved Stability and Therapeutic Efficacy. Nanomaterials 2017, 7, 165. [Google Scholar] [CrossRef]
- Kallum, M.K.; Stefanos, M.; Lakshminarayana, P.; Sara, E.S. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Paul, S.; Basak, S.; Ali, W. Zinc Stannate Nanostructure: Is It a New Class of Material for Multifunctional Cotton Textiles? ACS Omega 2019, 4, 21827–21838. [Google Scholar] [CrossRef]
- Soltani, N.; Saion, E.; Erfani, M.; Razaee, K.; Bahmanrokh, G.; Drummen, G.P.C.; Bahrami, A.; Hussein, M.Z. Influence of the Polyvinyl Pyrrolidone Concentration on Particle Size and Dispersion of ZnS Nanoparticles Synthesized by Microwave Irradiation. Int. J. Mol. Sci. 2012, 13, 12412–12427. [Google Scholar] [CrossRef]
- Saafi, I.; Dridi, R.; Mimouni, R.; Amlouk, A.; Yumak, A.; Boubaker, K.; Petkova, P.; Amlouk, M. Microstructural and optical properties of SnO2-ZnSnO3 ceramics. Ceram. Int. 2016, 42, 6273–6381. [Google Scholar] [CrossRef]
- Soltani, N.; Dehzangi, A.; Kharazmi, A.; Saion, E.; Yunus, W.M.M.; Majlis, B.Y.; Zare, M.R.; Gharibshahi, E.; Khalilzadeh, N. Structural, Optical and Electrical Properties of ZnS nanoparticles affecting by organic coating. Chalcogenide Lett. 2014, 11, 79–90. [Google Scholar]
- Saeidi, M.; Abrari, M.; Ahmadi, M. Fabrication of dye-sensitized solar cell based on mixed tin and zinc oxide nanoparticles. Appl. Phys. A 2019, 125, 409. [Google Scholar] [CrossRef]
- Turky, A.O.; Barhoum, A.; MohamedRashad, M.; Bechlany, M. Enhanced the structure and optical properties for ZnO/PVP noanofibres fabricated via electrospinning technique. J. Mater. Sci. Mater. Electron. 2017, 28, 17526–17532. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indhumathi, R.; Priya, A.S. Synthesis and Characterization of ZnSnO3/PVP Electrospun Composite Nanofibers. Mater. Proc. 2025, 25, 5. https://doi.org/10.3390/materproc2025025005
Indhumathi R, Priya AS. Synthesis and Characterization of ZnSnO3/PVP Electrospun Composite Nanofibers. Materials Proceedings. 2025; 25(1):5. https://doi.org/10.3390/materproc2025025005
Chicago/Turabian StyleIndhumathi, R., and A. Sathiya Priya. 2025. "Synthesis and Characterization of ZnSnO3/PVP Electrospun Composite Nanofibers" Materials Proceedings 25, no. 1: 5. https://doi.org/10.3390/materproc2025025005
APA StyleIndhumathi, R., & Priya, A. S. (2025). Synthesis and Characterization of ZnSnO3/PVP Electrospun Composite Nanofibers. Materials Proceedings, 25(1), 5. https://doi.org/10.3390/materproc2025025005



