Preparation and Characterization of NaYF4-Based Up-Conversion Nanoparticles for Solar Energy Storage Systems †
Abstract
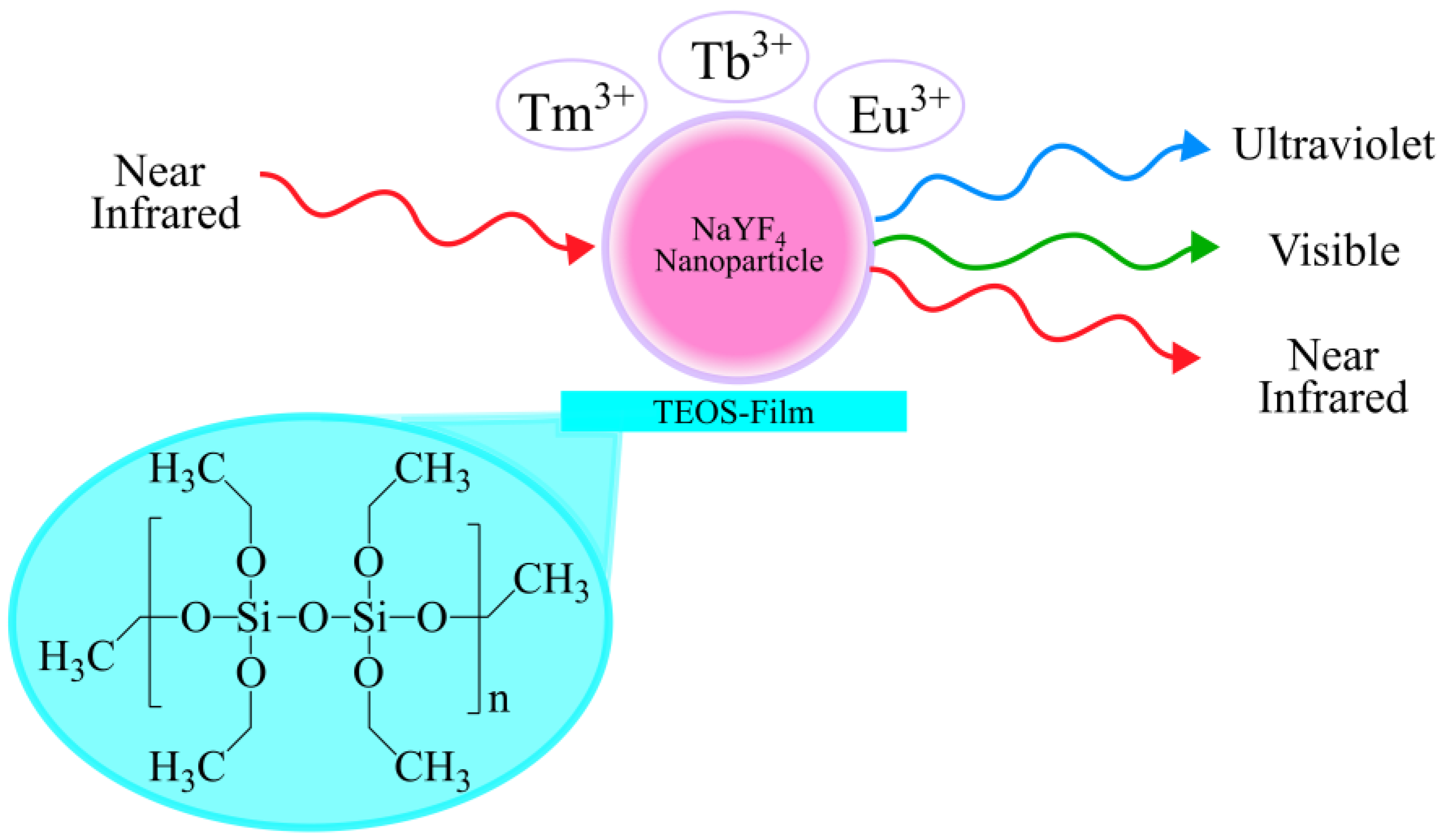
1. Introduction
2. Materials and Methods
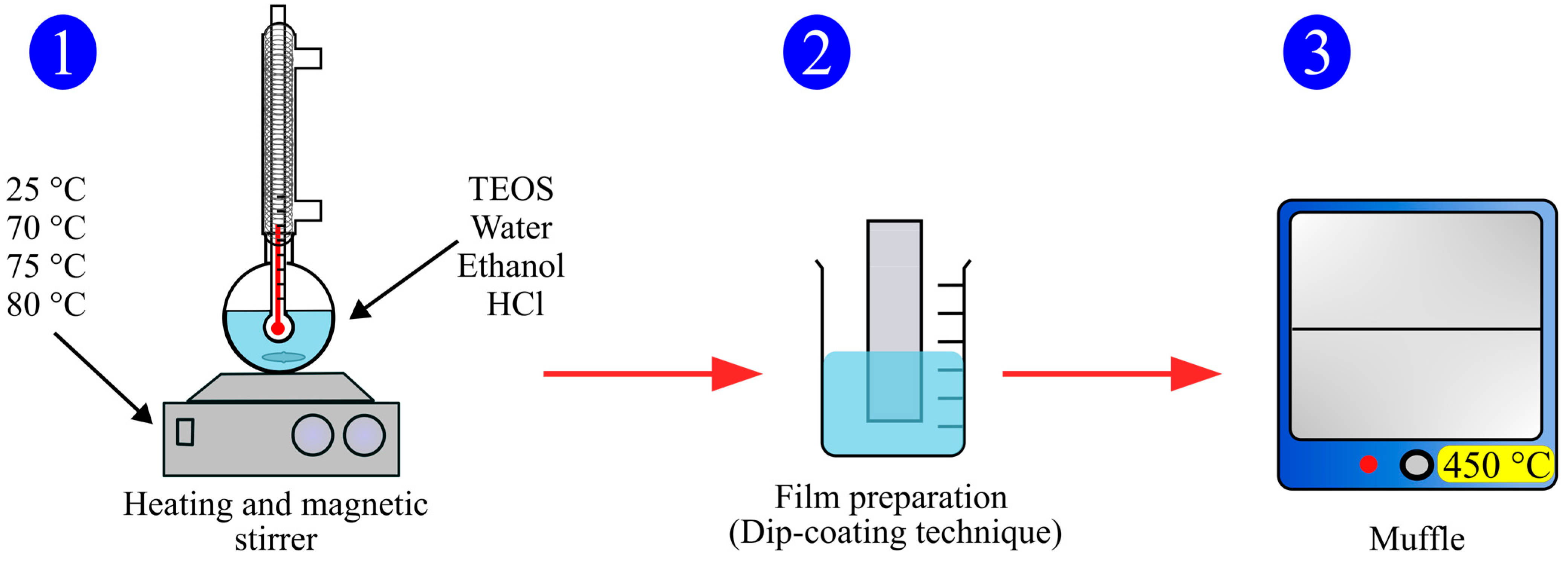
2.1. Synthesis of Sodium Yttrium Fluoride (NaYF4) Nanoparticles and TEOS Films
2.1.1. Synthesis of NaYF4 Up-Conversion Nanoparticles
2.1.2. Synthesis of Tetraethyl Orthosilicate (TEOS) Films
2.2. Characterization of (Sodium Yttrium Fluoride) NaYF4 Nanoparticles and TEOS Films
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. X-Ray Diffraction (XRD)
2.2.3. Multiphoton Confocal Microscopy (CM)
3. Preliminary Results
3.1. Characterization of NaYF4 Up-Conversion Nanoparticles with Different Heat-Treatment Times and TEOS Films Synthesized at Various pH Values and Temperatures
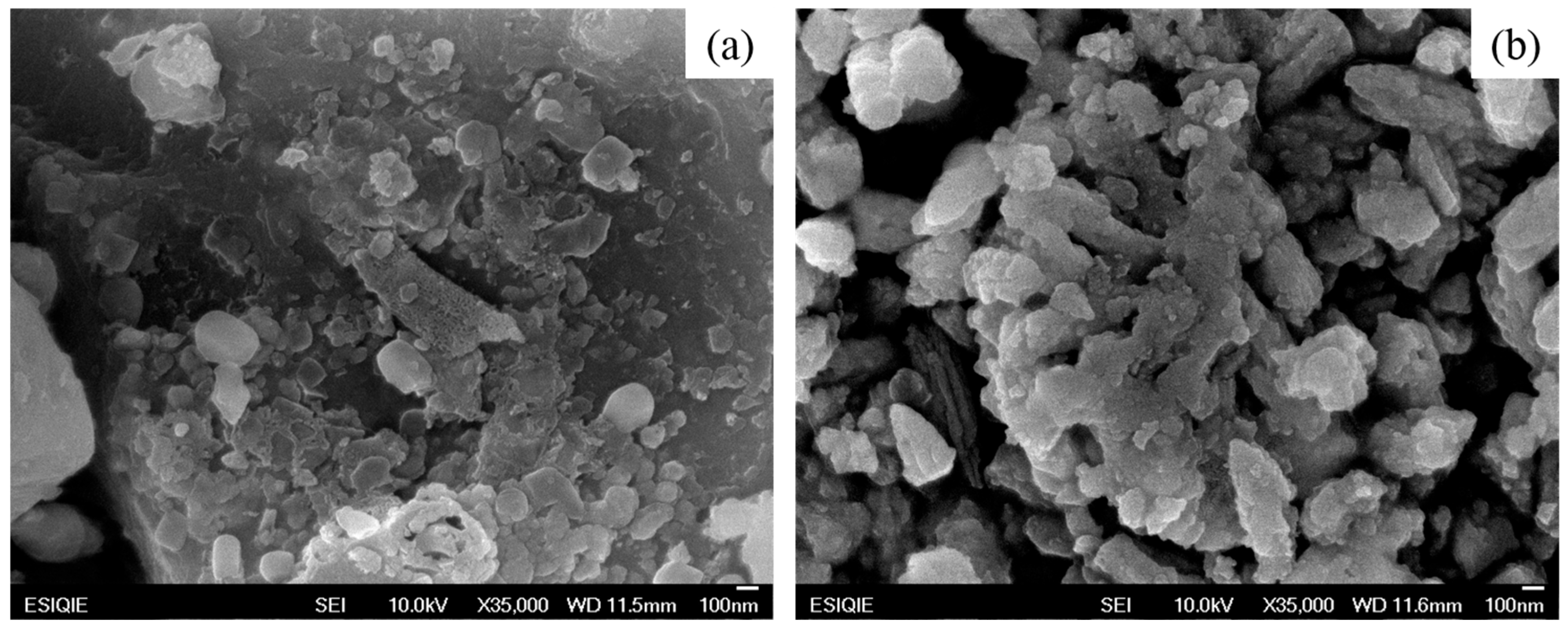
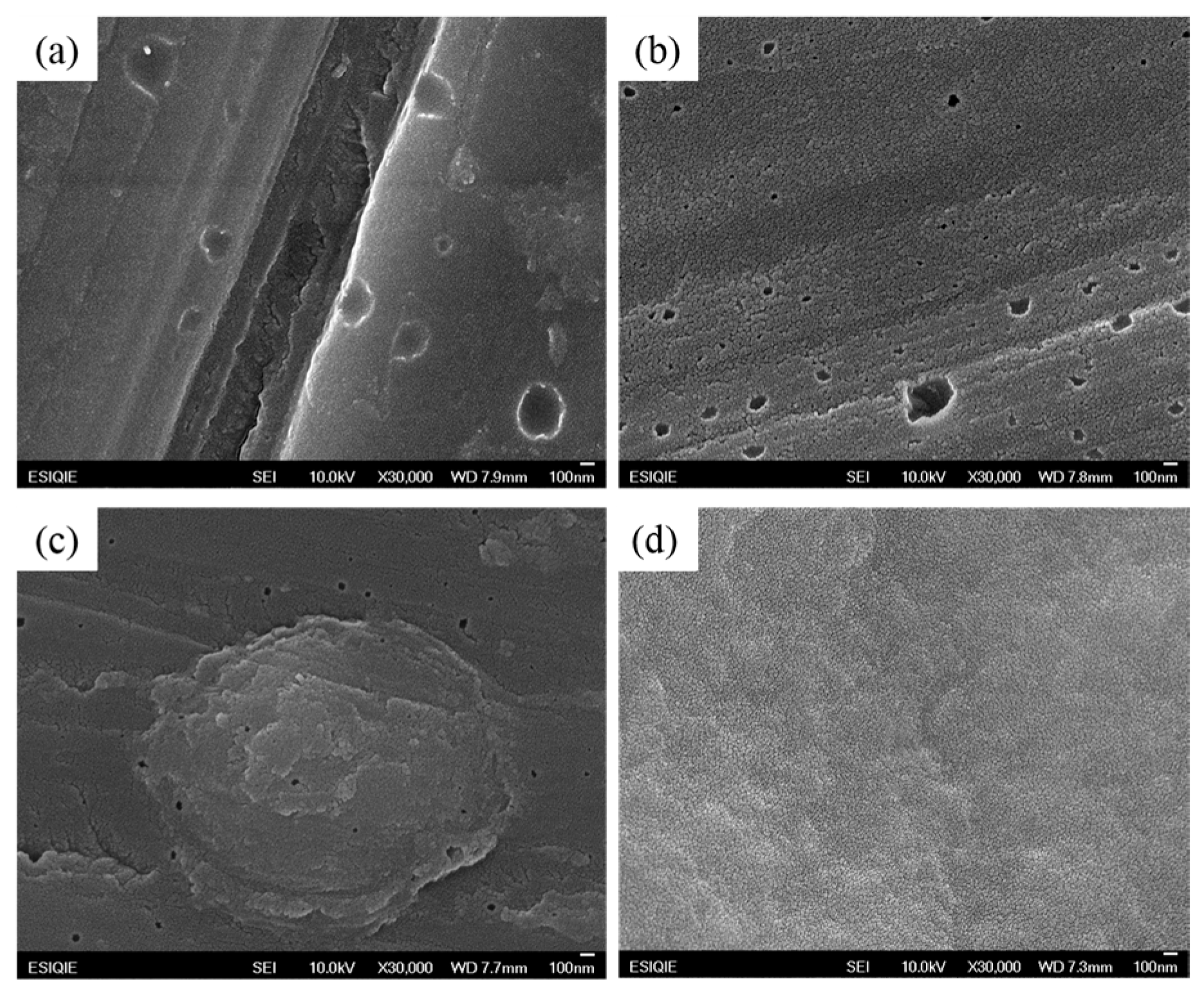
3.1.1. Scanning Electron Microscopy (SEM) of NaYF4 Up-Conversion Nanoparticles
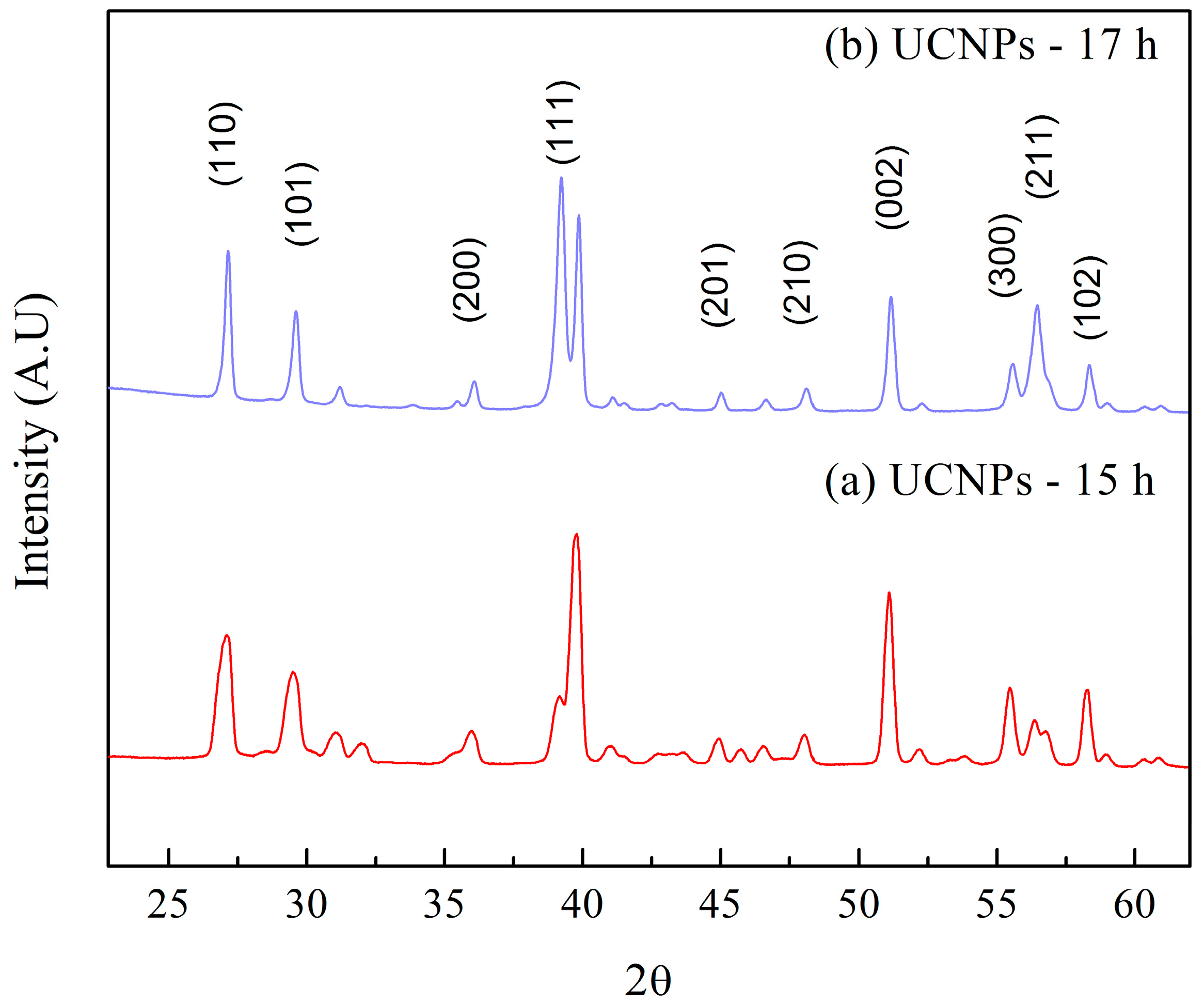
3.1.2. X-Ray Diffraction (XRD) Spectra of NaYF4 Up-Conversion Nanoparticles
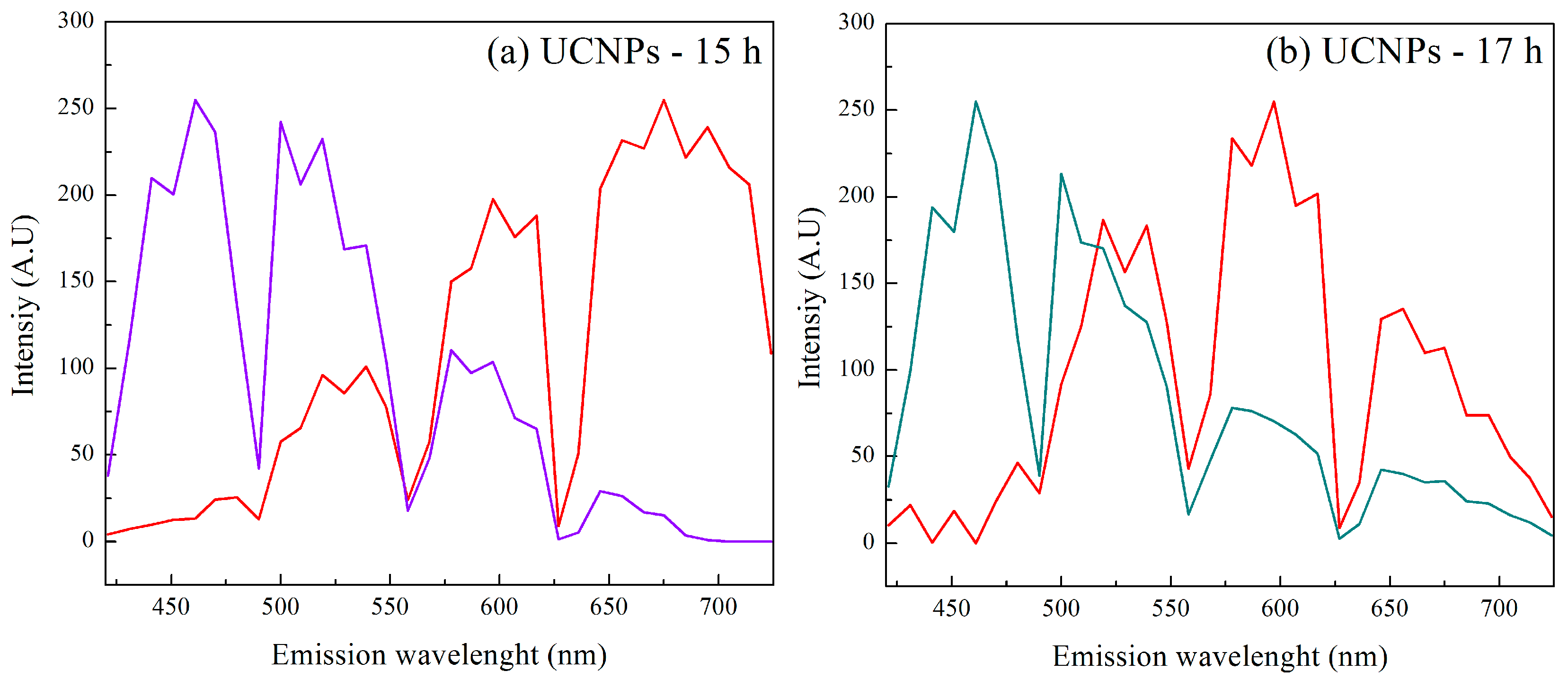
3.1.3. Multiphoton Confocal Microscopy (CM) of NaYF4 Up-Conversion Nanoparticles
3.1.4. Scanning Electron Microscopy (SEM) of TEOS Film Synthesized at Different pH and Temperatures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdolhosseini, M.; Zandsalimi, F.; Moghaddam, F.S.; Tavoosidana, G. A review on colorimetric assays for DNA virus detection. J. Virol. Methods 2022, 301, 114461. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Virgen, L.; Hernández-Martínez, M.A.; Martínez-Mejía, G.; Caro-Briones, R.; del Río, J.M.; Corea, M. Study of Thermodynamic and Rheological Properties of Sensitive Polymeric Nanoparticles as a Possible Application in the Oil Industry. J. Solut. Chem. 2024, 53, 5–27. [Google Scholar] [CrossRef]
- Ruiz-Virgen, L.; Hernández-Martínez, M.A.; Martínez-Mejía, G.; Caro-Briones, R.; Herbert-Pucheta, E.; el Río, J.M.; Corea, M. Analysis of Structural Changes of pH–Thermo-Responsive Nanoparticles in Polymeric Hydrogels. Gels 2024, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Manchanda, H.; Gupta, M. Recent advances in green synthesis, characterization and emerging applications of nanoparticles and derived nanofluids. Renew. Sustain. Energy Rev. 2026, 226, 116354. [Google Scholar] [CrossRef]
- Nadeem, J.; Dirk, L. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, G.; Shen, J.; Li, Z.; Zhang, Y.; Han, G. Upconversion Nanoparticles: A Versatile Solution to Multiscale Biological Imaging. Bioconjugate Chem. 2015, 26, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Sánchez, R.; Martinez-Calvo, E.A.; Sánchez-Pozos, M.; Corea, M. Review on polymer degradation by selective solar concentration using up-conversion nanoparticles. J. Polym. Res. 2023, 30, 296. [Google Scholar] [CrossRef]
- Vijay, K.; Irfan, A.; Hendrik, C.S.; Rakesh, S. Upconversion Nanoparticles (UCNPs) for Functional Applications, 1st ed.; Springer: Singapore, 2023; pp. 1–465. [Google Scholar]
- Khalid, H.; Chaudhry, A.A. Basics of Hydroxyapatite Structure, Synthesis, Properties, and Clinical Applications. In Biomedical Materials: Advances and Applications, 2nd ed.; Smith, J., Doe, R., Eds.; Springer: Berlin, Germany, 2020; Volume 4, pp. 154–196. [Google Scholar]
- Varanda, L.C.; Souza, C.G.S.d.; Perecin, C.J.; Moraes, D.A.d.; Queiróz, D.F.d.; Neves, H.R.; Souza Junior, J.B.; Silva, M.F.d.; Albers, R.F.; Silva, T.L.d. Inorganic and Organic–Inorganic Composite Nanoparticles with Potential Biomedical Applications: Synthesis Challenges for Enhanced Performance. In Materials for Biomedical Engineering: Bioactive Materials, Properties, and Applications, 1st ed.; Grumezescu, V., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–99. [Google Scholar]
- Yang, Z.; Xia, Z. Ceramics Phosphor Powders. In Advanced Ceramics for Energy Storage, Thermoelectrics and Photonics; Elsevier Series in Advanced Ceramic Materials; Cao, P., Chen, Z.-g., Xia, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 395–429. [Google Scholar]
- Zheng, K.; Boccaccini, A.R. Sol-gel processing of bioactive glass nanoparticles: A review. Adv. Colloid Interface Sci. 2017, 249, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Larry, L.; HenchJon, K.W. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Saheb Naher, H.; Al-Turaihi, B.A.H.; Mohammed, S.H.; Naser, S.M.; Albark, M.A.; Madlool, H.A.; Al-Marzoog, H.A.M.; Jalil, A.T. Upconversion Nanoparticles (UCNPs): Synthesis Methods, Imaging and Cancer Therapy. J. Drug Deliv. Sci. Technol. 2023, 80, 104175. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, J.; Zheng, K. Advances in highly doped upconversion nanoparticles. Nat. Commun. 2018, 9, 2415. [Google Scholar] [CrossRef] [PubMed]
- Beien, Z.; Rui, Q.; Lina, Y.; Yi, G. Real-time atomistic simulation of the Ostwald ripening of TiO2 supported Au nanoparticles. Nanoscale 2020, 12, 19142–19148. [Google Scholar] [CrossRef]
- Wei, Y.; Lu, F.; Zhang, X.; Chen, D. Synthesis and characterization of efficient near-infrared upconversion Yb and Tm codoped NaYF4 nanocrystal reporter. Alloys Compd. 2007, 427, 333–340. [Google Scholar] [CrossRef]
- Li, H.; Shi, X.; Li, X.; Zong, L. Size-tunable β-NaYF4:Yb/Er up-converting nanoparticles with a strong green emission synthesized by thermal decomposition. Opt. Mater. 2020, 108, 110144. [Google Scholar] [CrossRef]
- Chen, C.; Wang, F.; Wen, S.; Su, Q.P.; Wu, M.C.; Liu, Y.; Wang, B.; Li, D.; Shan, X.; Kianinia, M.; et al. Multi-photon near-infrared emission saturation nanoscopy using upconversion nanoparticles. Nat. Commun. 2018, 9, 3290. [Google Scholar] [CrossRef] [PubMed]
- Darmawan, A.; Munzakka, L.; Karlina, L.; Saputra, R.E.; Sriatun, S.; Astuti, Y.; Wahyuni, A.S. Pervaporation membrane for desalination derived from tetraethylorthosilicate-methyltriethoxysilane. J. Sol-Gel Sci. Technol. 2022, 101, 505–518. [Google Scholar] [CrossRef]
- Chang, K.-C.; Chen, Y.-K.; Chen, H. Fabrication of highly transparent and superhydrophobic silica-based surface by TEOS/PPG hybrid with adjustment of the pH value. Surf. Coat. Tech. 2008, 202, 3822–3831. [Google Scholar] [CrossRef]

| Chemicals | Source and Country | Molecular Weight (Mw) and Mass Fraction Purity | CAS No |
|---|---|---|---|
| Sodium fluoride (NaF) | Sigma-Aldrich; Bangalore; India. | Mw ~ 41.99 g mol−1; ≥ 99.0% | 7681-494 |
| Ethylenediaminetetraacetic acid (EDTA) | J.T. Baker; Mexico City; Mexico. | Mw ~ 372.24 g mol−1; ≥ 99.99% | 6381-92-6 |
| Tetraethyl orthosilicate (TEOS) | Sigma-Aldrich; Wuxi City; China. | Mw ~ 208.33 g mol−1; ≥ 98.0% | 78-10-14 |
| Yttrium oxide (Y2O3) | Sigma-Aldrich; Wuxi; China. | Mw ~ 225.81 g mol−1; ≥ 99.99% | 1314-36-9 |
| Thulium oxide (Tm2O3) | Sigma-Aldrich; Urbana; USA. | Mw ~ 385.87 g mol−1; ≥ 99.99% | 12036-44-1 |
| Ytterbium oxide (Yb2O3) | Sigma-Aldrich; Wuxi; China. | Mw ~ 394.08 g mol−1; ≥ 99.99% | 1314-37-0 |
| Hydrochloric acid (HCl) | Herschi Trading; Mexico City; Mexico. | Mw ~ 36.46 g mol−1; ≥ 36.50% a | 7647-01-0 |
| Distilled water (H2O) | Isse Labs. S.A. de C.V.; López Mateos City; Mexico. | Mw ~ 18.02 g mol−1; a | 7732-18-5 |
| Ethanol (C2H5OH) | D’Mik; Los Reyes Acaquilpan; Mexico. | Mw ~ 46.07 g mol−1; ≥ 96.0% | 64-17-5 |
| Compounds | Mass (g) | ||||
|---|---|---|---|---|---|
| Yttrium oxide (Y2O3) | 0.3 | ||||
| Thulium oxide (Tm2O3) | 0.015 | ||||
| Ytterbium oxide (Yb2O3) | 0.1 | ||||
| Ethylenediaminetetraacetic acid (EDTA) | 1.1 | ||||
| Hydrochloric acid (HCl) | 8.0 | 0.3 | 3.4 | 10 | |
| Sodium fluoride (NaF) | 1 | ||||
| Distilled water (H2O) | 30 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manjarrez-Arellano, J.J.; Hernandez-Martinez, M.A.; Caro-Briones, R.; Martínez-Mejía, G.; Ruiz-Virgen, L.; del Río, J.M.; Sánchez-Pozos, M.; Corea, M. Preparation and Characterization of NaYF4-Based Up-Conversion Nanoparticles for Solar Energy Storage Systems. Mater. Proc. 2025, 25, 16. https://doi.org/10.3390/materproc2025025016
Manjarrez-Arellano JJ, Hernandez-Martinez MA, Caro-Briones R, Martínez-Mejía G, Ruiz-Virgen L, del Río JM, Sánchez-Pozos M, Corea M. Preparation and Characterization of NaYF4-Based Up-Conversion Nanoparticles for Solar Energy Storage Systems. Materials Proceedings. 2025; 25(1):16. https://doi.org/10.3390/materproc2025025016
Chicago/Turabian StyleManjarrez-Arellano, José Joaquín, Miguel A. Hernandez-Martinez, Rubén Caro-Briones, Gabriela Martínez-Mejía, Lazaro Ruiz-Virgen, José Manuel del Río, Miriam Sánchez-Pozos, and Mónica Corea. 2025. "Preparation and Characterization of NaYF4-Based Up-Conversion Nanoparticles for Solar Energy Storage Systems" Materials Proceedings 25, no. 1: 16. https://doi.org/10.3390/materproc2025025016
APA StyleManjarrez-Arellano, J. J., Hernandez-Martinez, M. A., Caro-Briones, R., Martínez-Mejía, G., Ruiz-Virgen, L., del Río, J. M., Sánchez-Pozos, M., & Corea, M. (2025). Preparation and Characterization of NaYF4-Based Up-Conversion Nanoparticles for Solar Energy Storage Systems. Materials Proceedings, 25(1), 16. https://doi.org/10.3390/materproc2025025016





