A Semi-Automated Framework for Standardized Vertebral Measurement with Enhanced Reproducibility in Lumbar Spine MRI Analysis †
Abstract
1. Introduction
2. Methodology
2.1. Dataset
2.2. File Handling and Preprocessing
2.3. Mathematical Modeling
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cappetti, N.; Angelo, L.D.; Fontana, C.; Marzola, A. A computer-based method for the automatic identification of the dimensional features of human cervical vertebrae. Comput. Methods Programs Biomed. Update 2025, 7, 100175. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Liu, T.; Wang, M.; Sun, M.; Song, S.; Fan, W.; Huang, G. Anatomical prior-based vertebral landmark detection for spinal disorder diagnosis. Artif. Intell. Med. 2025, 159, 103011. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ngiam, K.; Teo, H. Role of Artificial Intelligence Applications in Real-Life Clinical Practice: Systematic Review. J. Med. Internet Res. 2020, 23, e25759. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Brennan, M.; Mohamed, B.; Rashidi, P.; Modave, F.; Tighe, P. Accessing Artificial Intelligence for Clinical Decision-Making. Front. Digit. Health 2021, 3, 645232. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, I.; Rundo, L.; Codari, M.; Di Leo, G.; Salvatore, C.; Interlenghi, M.; Gallivanone, F.; Cozzi, A.; D’Amico, N.; Sardanelli, F. AI applications to medical images: From machine learning to deep learning. Phys. Medica Int. J. Devoted Appl. Phys. Med. Biol. 2021, 83, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.; Hinton, G.; Wells, A.; Van Der Veken, J.; Bajger, M.; Lee, G.; Liu, Y.; Chong, C.; Poonnoose, S.; Agzarian, M.; et al. Imaging evaluation of a proposed 3D generative model for MRI to CT translation in the lumbar spine. Spine J. Off. J. N. Am. Spine Soc. 2023, 23, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Graaf, J.; Hooff, M.; Buckens, C.; Rutten, M.; Susante, J.; Kroeze, R.; Kleuver, M.; Ginneken, B.; Lessmann, N. Lumbar spine segmentation in MR images: A dataset and a public benchmark. Sci. Data 2023, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Masood, R.; Hassan, T.; Raja, H.; Hassan, B.; Dias, J.; Werghi, N. A Composite Dataset of Lumbar Spine Images with Mid-Sagittal View Annotations and Clinically Significant Spinal Measurements. In Proceedings of the 2022 2nd International Conference on Digital Futures and Transformative Technologies (ICoDT2), Rawalpindi, Pakistan, 24–26 May 2022; pp. 1–5. [Google Scholar] [CrossRef]
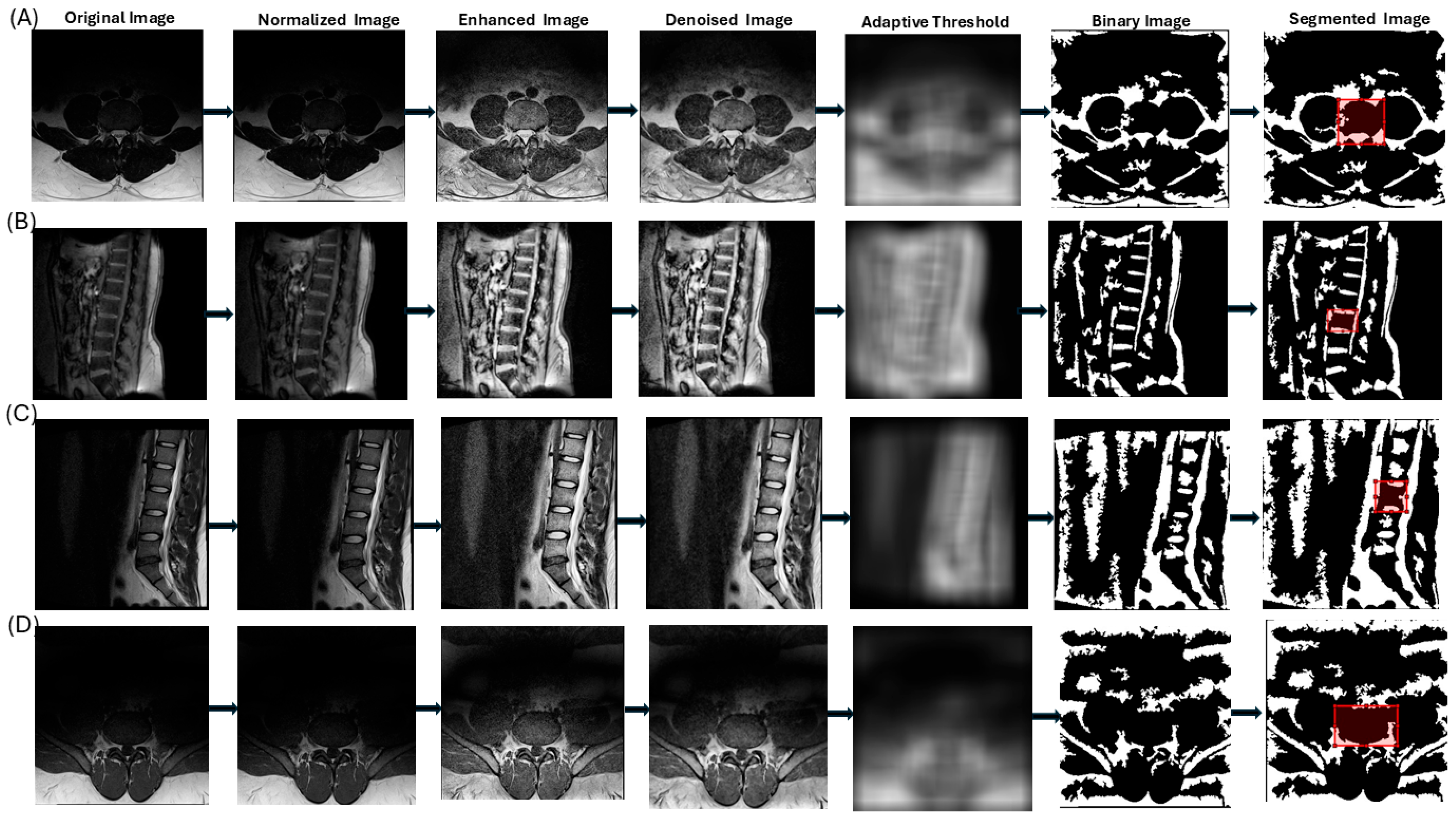
| Step | Description | Purpose | MATLAB Command |
|---|---|---|---|
| 1 | Convert IMA file to DICOM format | Standardize data and extract metadata (e.g., pixel spacing, slice thickness) | dicomread, dicominfo, dicomwrite |
| 2 | Convert image to grayscale | Focus on intensity variations, eliminating color channels | rgb2gray |
| 3 | Normalize image intensities to range (0, 1) | Ensure consistency across images for uniform processing | mat2gray |
| 4 | Enhance contrast by locally redistributing intensities in small regions | Improve visibility of vertebral boundaries with adaptive contrast adjustment | adapthisteq |
| 5 | Reduce noise by replacing each pixel with the median of its 3 × 3 neighborhood | Remove small-scale noise while preserving vertebral edges | medfilt2 |
| 6 | Segment image using adaptive thresholding based on local intensity | Create a binary image, assuming vertebral structures are brighter than the background | adaptthresh |
| 7 | Correct polarity if foreground is darker than background | Ensure vertebral structures are correctly identified as foreground | imbinarize |
| 8 | Apply watershed thresholding to refine segmentation | Delineate boundaries between vertebral structures using a topographic flooding approach | bwdist, watershed |
| 9 | Apply morphological operations to refine segmentation | Remove noise, close gaps, eliminate small objects, and fill holes for structural integrity | imopen, imclose, bwareaopen, imfill |
| 10 | Allow user to draw a rectangular ROI on the segmented image | Enable user-guided selection of the vertebral body of interest | drawrectangle |
| 11 | Create a mask from the ROI and combine it with the segmented image | Isolate the vertebral structure within the user-defined ROI | Logical AND operation |
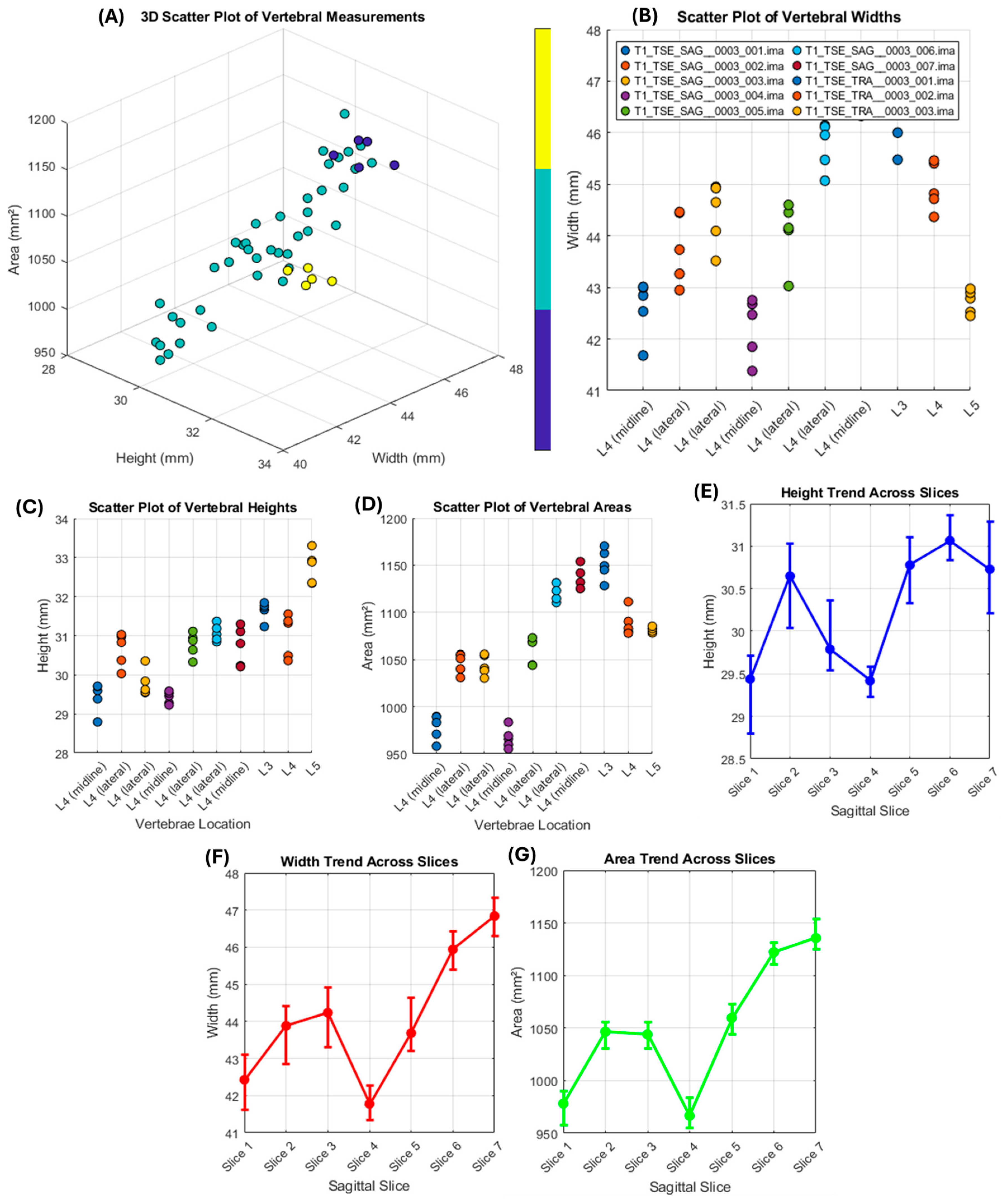
| File Name | Vertebrae Location | Height (mm) | Width (mm) | Area (mm2) |
|---|---|---|---|---|
| T1_TSE_SAG__0003_001.ima | L4 (midline) | 29.23 | 42.31 | 971.37 |
| T1_TSE_SAG__0003_002.ima | L4 (lateral) | 30.47 | 43.65 | 1044.62 |
| T1_TSE_SAG__0003_003.ima | L4 (lateral) | 30.11 | 44.12 | 1043.37 |
| T1_TSE_SAG__0003_004.ima | L4 (midline) | 29.29 | 41.98 | 965.77 |
| T1_TSE_SAG__0003_005.ima | L4 (lateral) | 30.63 | 43.84 | 1054.77 |
| T1_TSE_SAG__0003_006.ima | L4 (lateral) | 31.22 | 45.67 | 1119.92 |
| T1_TSE_SAG__0003_007.ima | L4 (midline) | 30.76 | 47.21 | 1140.47 |
| T1_TSE_TRA__0003_001.ima | L3 | 31.53 | 46.34 | 1147.62 |
| T1_TSE_TRA__0003_002.ima | L4 | 30.98 | 44.93 | 1093.24 |
| T1_TSE_TRA__0003_003.ima | L5 | 32.82 | 42.54 | 1096.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masrur, M.H.; Khalid, R.T.; Wara, K.U.; Alber, A.; Ahmad, F.; Bibi, Z.; Hussain, J. A Semi-Automated Framework for Standardized Vertebral Measurement with Enhanced Reproducibility in Lumbar Spine MRI Analysis. Mater. Proc. 2025, 23, 5. https://doi.org/10.3390/materproc2025023005
Masrur MH, Khalid RT, Wara KU, Alber A, Ahmad F, Bibi Z, Hussain J. A Semi-Automated Framework for Standardized Vertebral Measurement with Enhanced Reproducibility in Lumbar Spine MRI Analysis. Materials Proceedings. 2025; 23(1):5. https://doi.org/10.3390/materproc2025023005
Chicago/Turabian StyleMasrur, Muhammad Hasan, Rana Talha Khalid, Khair Ul Wara, Abdul Alber, Faizan Ahmad, Zainab Bibi, and Jawad Hussain. 2025. "A Semi-Automated Framework for Standardized Vertebral Measurement with Enhanced Reproducibility in Lumbar Spine MRI Analysis" Materials Proceedings 23, no. 1: 5. https://doi.org/10.3390/materproc2025023005
APA StyleMasrur, M. H., Khalid, R. T., Wara, K. U., Alber, A., Ahmad, F., Bibi, Z., & Hussain, J. (2025). A Semi-Automated Framework for Standardized Vertebral Measurement with Enhanced Reproducibility in Lumbar Spine MRI Analysis. Materials Proceedings, 23(1), 5. https://doi.org/10.3390/materproc2025023005





