1. Introduction
The growing worldwide need for efficient and sustainable energy storage mechanisms underlines the imperative demand for innovative materials. Transition metal sulfides, well known for their remarkable electrical conductivity, excellent energy density, economic viability, mechanical strength, and ease of synthetic pathways, have become hopeful contenders [
1,
2]. Iron–cobalt sulfide (FeCoS) has become one of the promising contenders among them. The synergistic effect of iron and cobalt cations in the FeCoS structure is expected to provide excellent electrochemical performance, and thus it is an interesting material for future energy storage devices [
3].
Many researchers favor transition metal sulfides (TMSs) from Bi, Co, Cd, Cu, Ni, and Fe due to their high specific capacitance, abundance, and feasibility. They outperform oxides and hydroxides [
4]. This study focuses on Fe- and Co-based sulfides for their stable and conductive structures [
5], high theoretical capacitance, fast Faradaic redox reactions, and ternary synergistic effects [
6]. Further, optimizing TMS electrodes requires composite engineering and morphological control [
7]. However, bimetallic Fe-Co sulfides remain understudied in energy storage, necessitating further exploration of Fe-Co sulfide composites. A synergistic multicomponent approach enhances ionic/electronic conductivity, boosting energy storage potential [
8]. Multicomponent TMSs surpass single-component sulfides in conductivity, stability, capacity, cycling performance, and efficiency, making them more viable for energy storage [
8].
The optimization of FeCoS/rGO composites for energy storage involves the controlled morphology and composition of FeCoS via customized synthesis. rGO increases conductivity and cushions volume changes, enhancing stability [
9]. These composites hold potential in supercapacitors, lithium ion, and sodium ion batteries. Future work is aimed at Fe/Co ratio optimization, rGO content, and new synthesis routes to further improve performance [
1]. To further improve the electrochemical property of FeCoS, especially for supercapacitor performance, the incorporation of reduced graphene oxide (rGO) is investigated. rGO, with high surface area, good electrical conductivity, and mechanical flexibility, achieves a synergistic effect when combined with FeCoS [
9]. This composite material exhibited superior charge storage properties, enhanced rate capability, and better cycle durability, making it a good material for advanced energy storage devices [
10].
2. Materials and Methods
All the chemicals were bought from Sigma-Aldrich (Saint Louis, MO, USA): ferric chloride hexahydrate (FeCl3∙6H2O; ≥76%), cobalt chloride hexahydrate (CoCl2∙6H2O; ≥99%), and sodium sulfide nonahydrate (Na2S∙9H2O; ≥99.99%). For enhanced electrochemical performance, a composite was prepared with reduced graphene oxide (rGO). Deionized water and ethanol, sourced from Sigma Scientific (Islamabad, Pakistan), were used during the precursor dissolution and washing. In synthesizing the Fe0.8Co0.2S/rGO composite, a mass ratio of 10:1 was used for Fe0.8Co0.2S and rGO. FeCl3.6H2O and CoCl2.6H2O, amounting to 0.0223 moles, were dissolved in 100 mL deionized water under stirring. Subsequently, 5.52 g of Na2S·9H2O was added slowly, allowing for the in situ formation of Fe-Co-S by the replacement of Na+ with Fe+2 and Co+2 ions at 70 °C. For even dispersion and agglomeration prevention, the reaction product was ultrasonicated. The material was then purified by washing and drying at 100 °C. Lastly, the resulting powder was thermally annealed at 400 °C using a ramped heating rate of 2 °C/min for four hours to maximize its crystallinity and electrochemical performance.
After material synthesis, iron copper sulfide (Fe0.8Cu0.2S) and the nanocomposite slurries were made. For the A1 Fe0.8Co0.2S electrode, 12 mg of active material was mixed with 30 µL of polyvinylidene fluoride (PVDF) and 40 µL of N-methyl-2-pyrrolidone (NMP) in a slurry tube. The mixture was stirred at 360 rpm for 8 h. In the case of the Fe0.8Cu0.2S/rGO (10:1) electrode (A2), 10.8 mg of the active material and 1.2 mg of reduced graphene oxide (rGO) were put into a slurry tube consisting of 30 µL of PVDF and 40 µL of NMP and stirred in an identical manner at 360 rpm for 8 h. Every prepared slurry was then coated onto individual nickel foam substrates and dried next to an oven at 80 °C for 10 h. The particles synthesized were characterized by scanning electron microscopy (SEM) and energy-dispersive X-ray analysis (EDS) through SEM (Carl Zeiss Evo 15, Jena, Germany), X-ray diffraction (XRD) (AXRD LPD, Proto, UK), and electrochemical analysis via a potentiostat (Gamry Instruments, Reference 3000, Warminster, PA, USA).
3. Results and Discussion
3.1. XRD Analysis
X-ray diffraction analysis was used to verify the phases of the synthesized materials by correlating the diffraction peaks to their respective standard JCPDS card numbers. X-ray diffraction (XRD) analysis identified clear crystalline phases for the two synthesized materials. For the first phase, referring to the Fe
0.8Co
0.2S sample, diffraction peaks at 2θ angles of 19, 28, 31, 33, 35, 45, 64, and 66 degrees were indicated. These peaks suggest the existence of certain crystallographic planes in the Fe
0.8Co
0.2S structure. In the second phase, corresponding to the Fe
0.8Co
0.2S/rGO composite, the XRD pattern revealed peaks at 2θ values of 19, 28, 31, 33, 35, 40, and 63 degrees, corresponding to the Fe
0.8Co
0.2S phase. Notably, a new peak was observed at 2θ = 24 degrees, characteristic of reduced graphene oxide (rGO). This indicates a successful integration of the rGO into the composite structure. The minimal shifts and intensity change in the Fe
0.8Co
0.2S peaks between the two phases could indicate minor changes in the crystalline structure or particle size because of the incorporation of the rGO. The identification of the rGO peak indicates the hybrid formation as shown in
Figure 1.
3.2. SEM
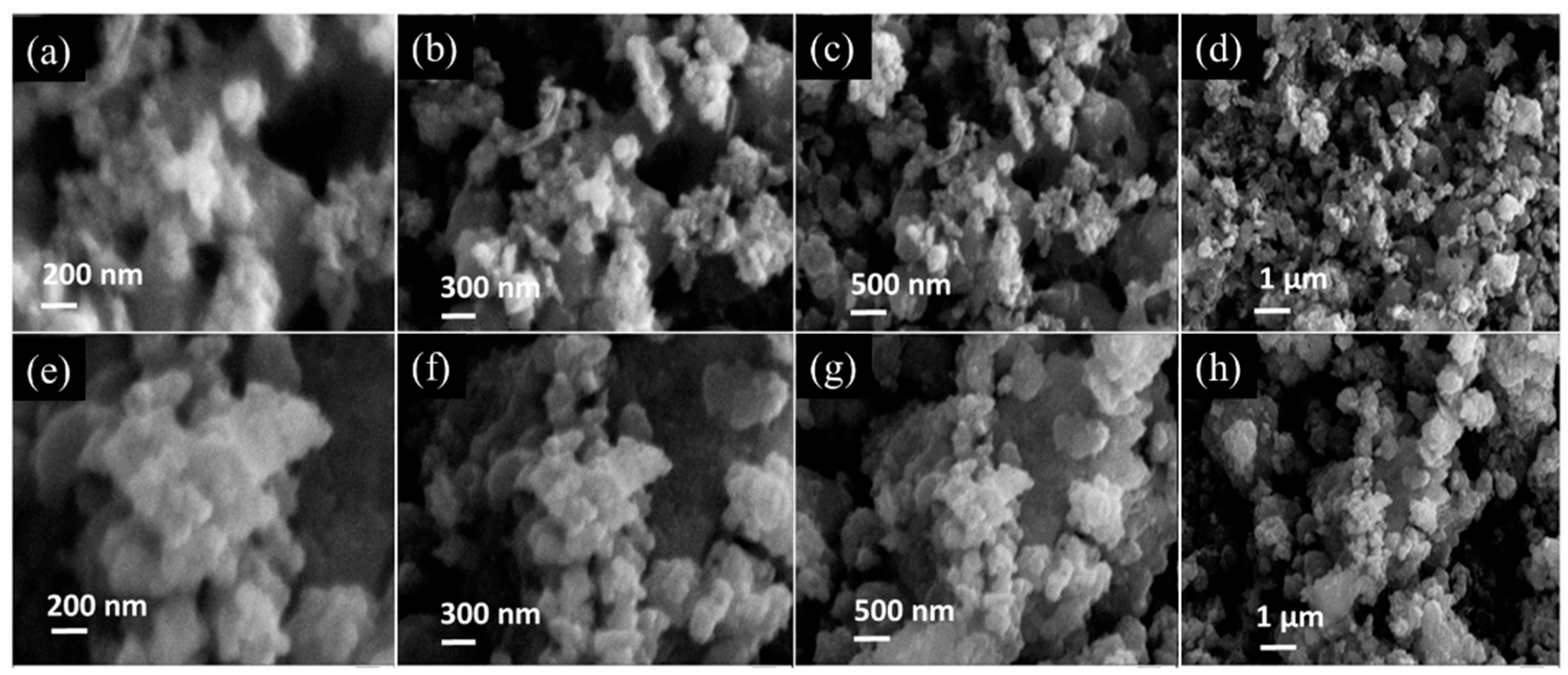
Scanning electron microscopy (SEM) characterization, as shown in
Figure 2a–d, showed that Fe
0.8Co
0.2S had an aggregated morphology of elongated and irregular structures. Upon the addition of reduced graphene oxide (RGO), a visible change in the morphology of the material was witnessed, as illustrated in
Figure 2e,f. The successful formation of the composite material is substantiated by the presence of RGO sheets, upon which aggregated Fe
0.8Co
0.2S particles are distributed. This structural modification is posited to enhance the feasibility of the Fe
0.8Co
0.2S/RGO composite as a material for energy storage applications.
3.3. EDS Elemental Mapping
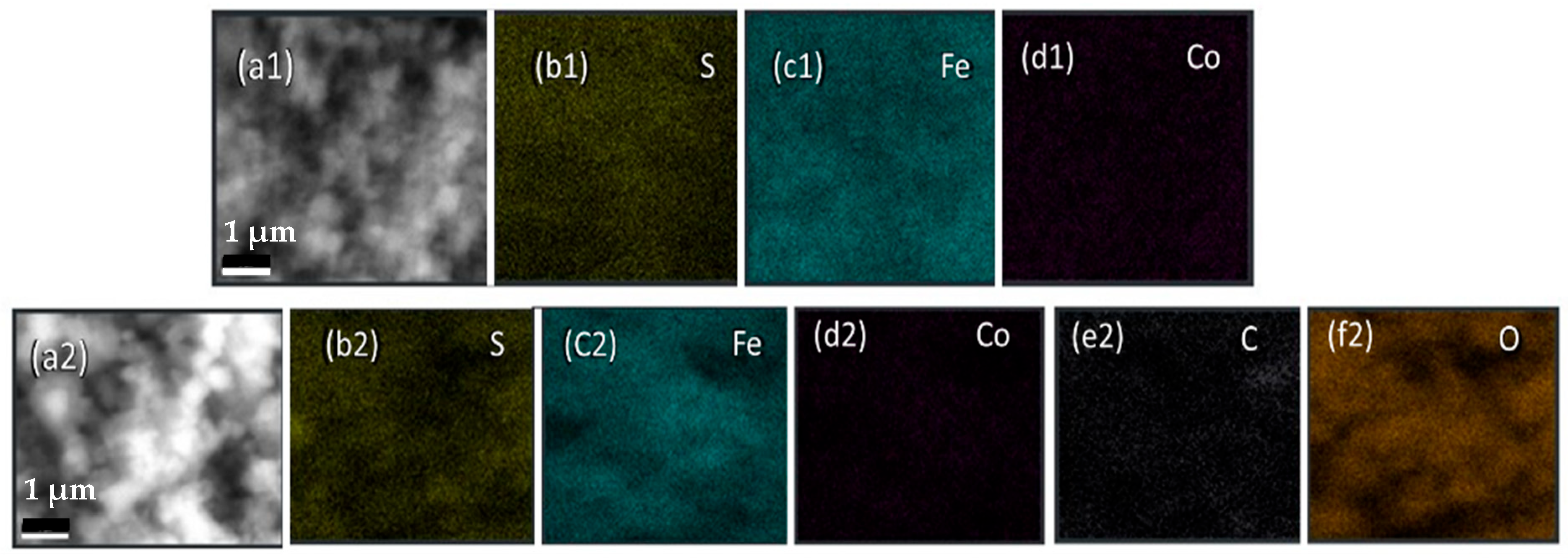
Elemental composition was determined via energy-dispersive X-ray spectroscopy (EDX). The EDX spectrum of Fe
0.8Co
0.2S is displayed in
Figure 3, validating the presence of iron, cobalt, and sulfur, confirming the successful synthesis. Minor peaks, likely artifacts of the coating, were also detected. Conversely, the EDX spectrum of Fe
0.8Co
0.2S/RGO, presented in
Figure 3, exhibited additional peaks corresponding to carbon. This observation confirms the successful integration of reduced graphene oxide within the composite, a factor anticipated to positively influence its electrochemical performance.
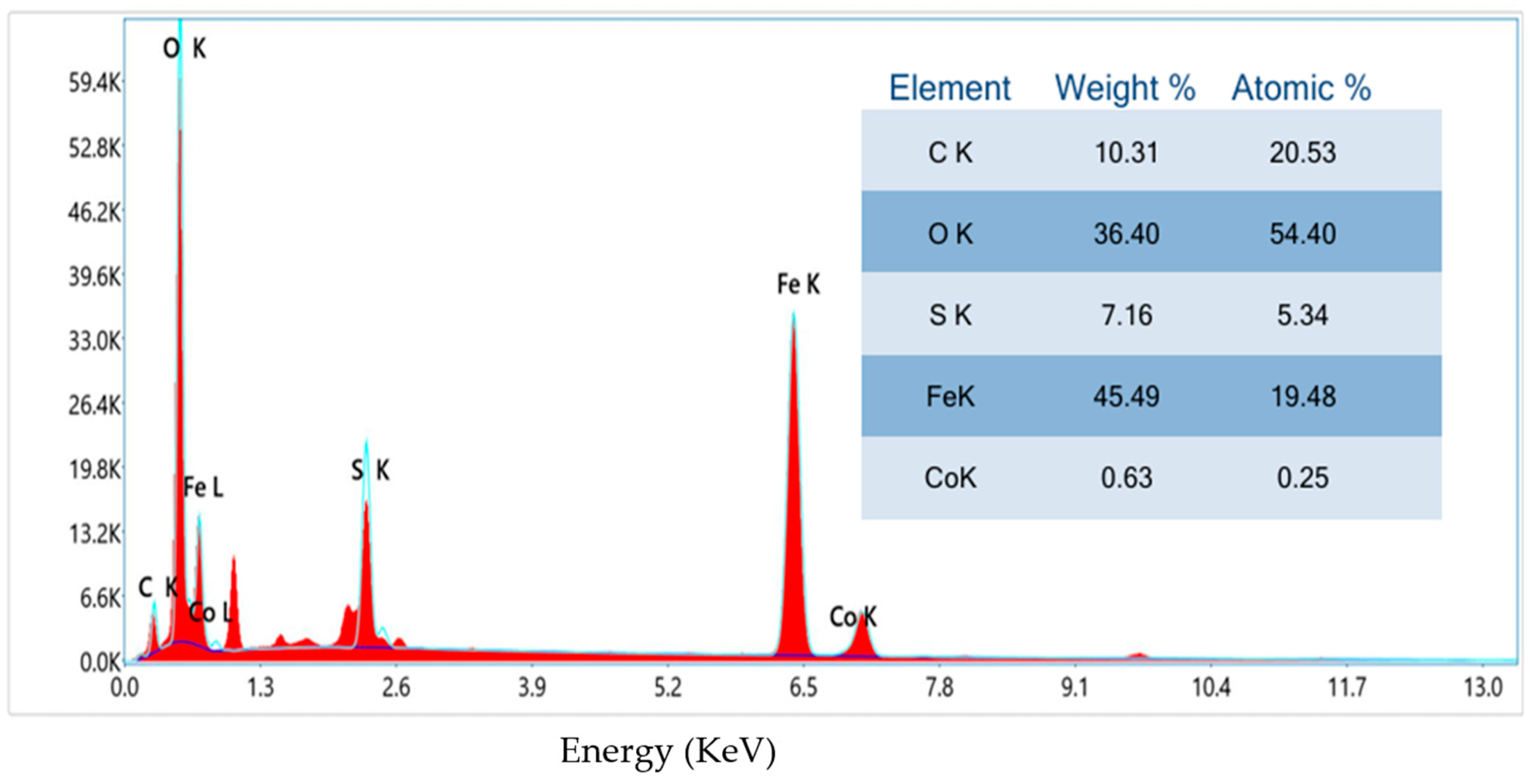
3.4. EDX Spectra
The elemental composition of the samples was analyzed using EDX. The Fe
0.8Co
0.2S sample (
Figure 4) exhibited characteristic peaks for iron, cobalt, and sulfur, confirming its formation. Additional peaks were noted, likely due to the coating. The EDX spectrum of Fe
0.8Co
0.2S/RGO (
Figure 5) further confirmed the successful synthesis of the composite by showing the additional peak of carbon, indicative of the presence of RGO, which is expected to improve electrochemical properties.
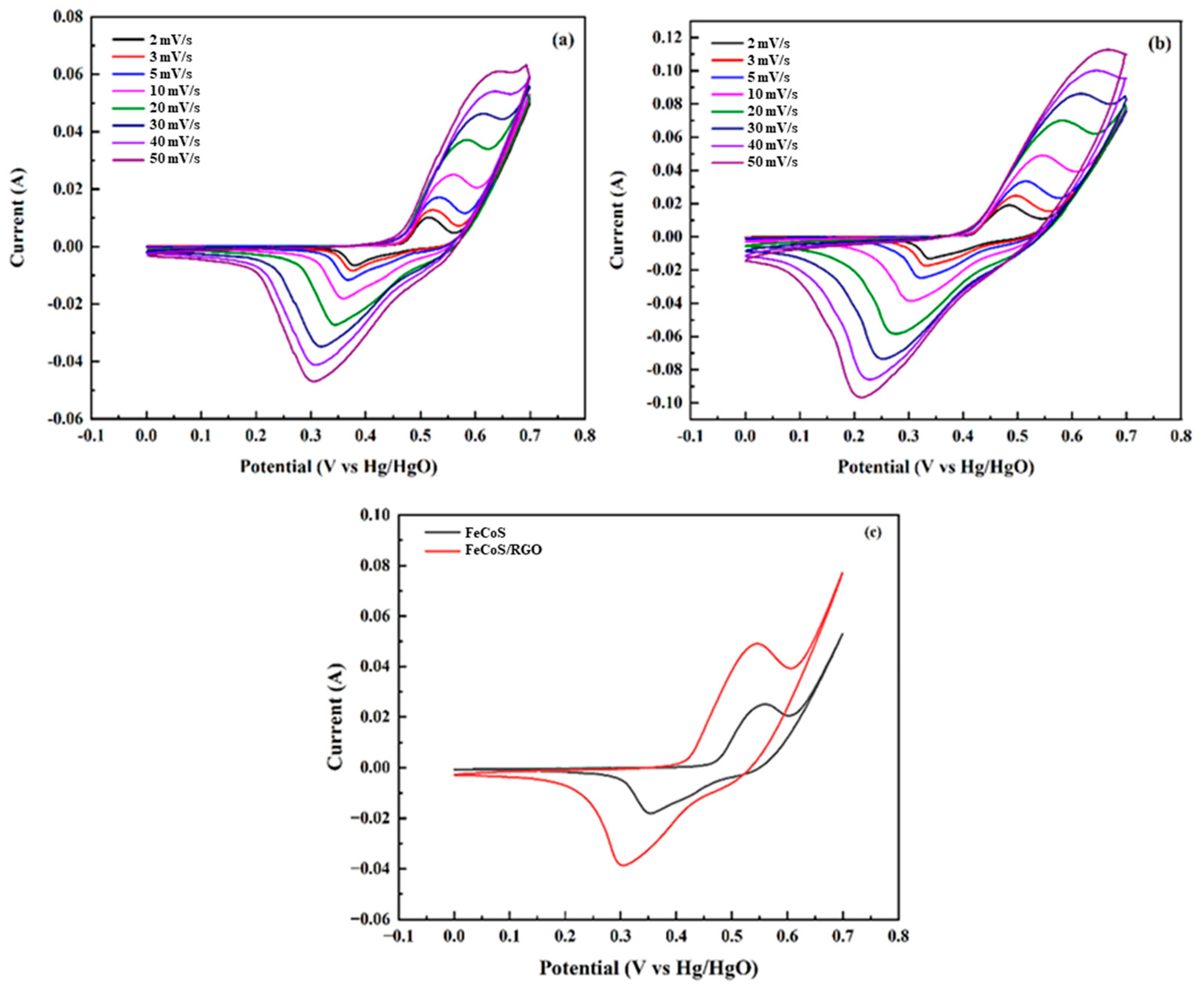
3.5. Cyclic Voltammetry Analysis
Electrochemical performance was assessed via cyclic voltammetry across a range of scan rates, from 2 to 50 mV/s. The Fe
0.8Co
0.2S electrode (P1), as shown in
Figure 5, displayed distinct redox peaks, signifying Faradaic reactions. At 10 mV/s, P1 exhibited an anodic peak at +25 mA and a cathodic peak at −18 Ma, see
Figure 6a. In contrast, the Fe
0.8Co
0.2S/RGO electrode (P2), depicted in
Figure 6b, showed an anodic peak of +49 mA and a cathodic peak of −39 mA at the same 10 mV/s scan rate. A clear enlargement of the area under the curve was observed for P2, indicating a substantial increase in capacitance. A comparative visualization of these specific capacitance values is provided in
Figure 6c.
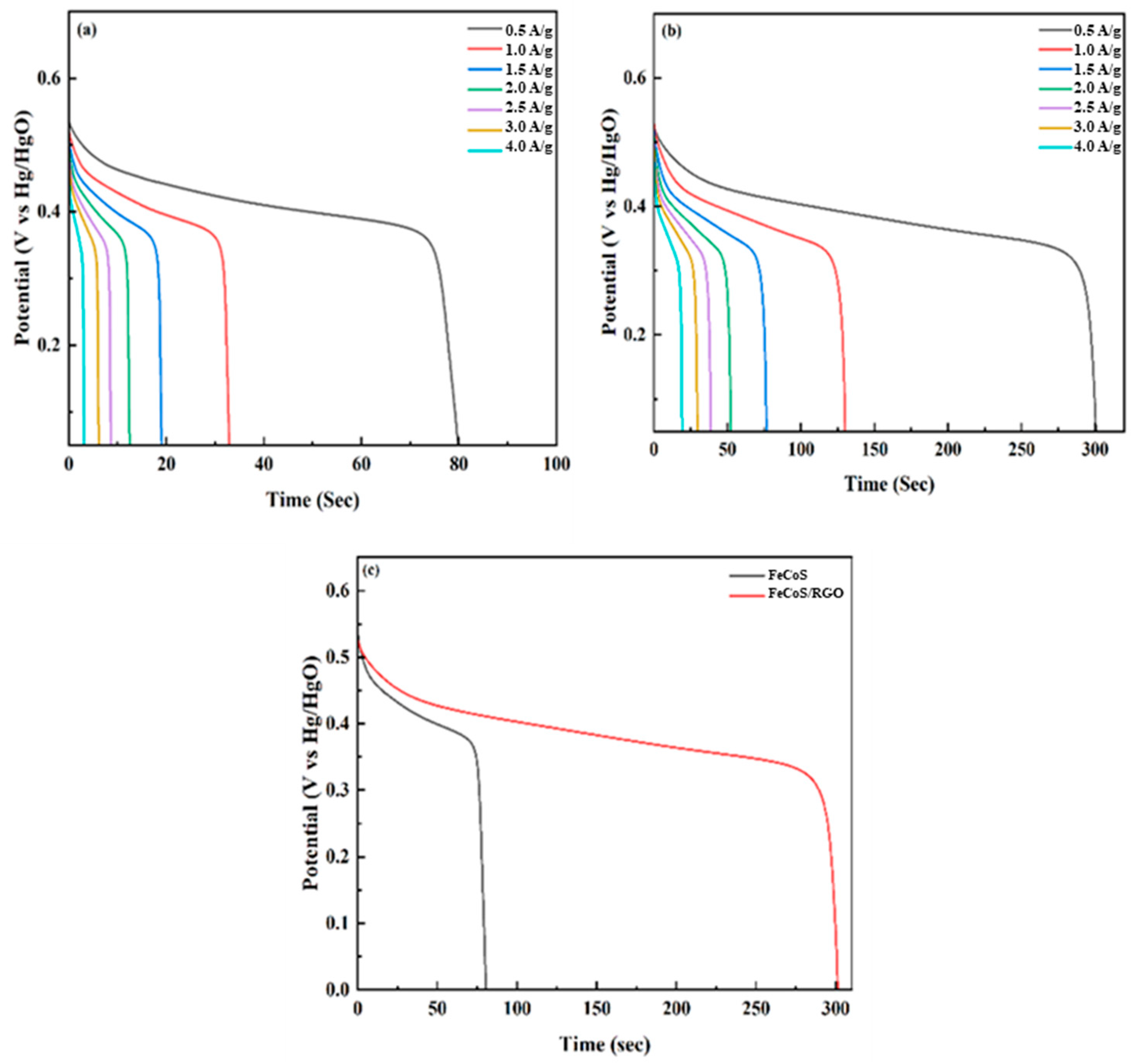
3.6. GCD Analysis
The galvanostatic charge–discharge (GCD) profiles provide insights into the charge and discharge mechanisms of the electrode material. Equation (1) is used to determine the specific capacity during the discharge process. In this equation, I/m represents the current density and ∆
t denotes the time taken for discharge:
Subsequently, the specific capacitance and energy density was calculated by incorporating the value obtained from Equation (1) into the following expression:
The GCD profiles, illustrated in
Figure 7a,b, demonstrate the electrochemical behavior of the electrodes across current densities from 0.5 to 4 A/g within a 0 to 0.5 V potential window. Notably, the discharge time and specific capacitance increased significantly from 78 s to 300 s and 74.3 F/g to 285.7 F/g (calculated using Equations (1) and (2)), respectively, representing a 283% improvement; see
Figure 7c. Furthermore, the energy density rose from 2.84 Wh/kg to 10.9 Wh/kg—a 284% enhancement—demonstrating the composite electrode’s superior energy storage performance.
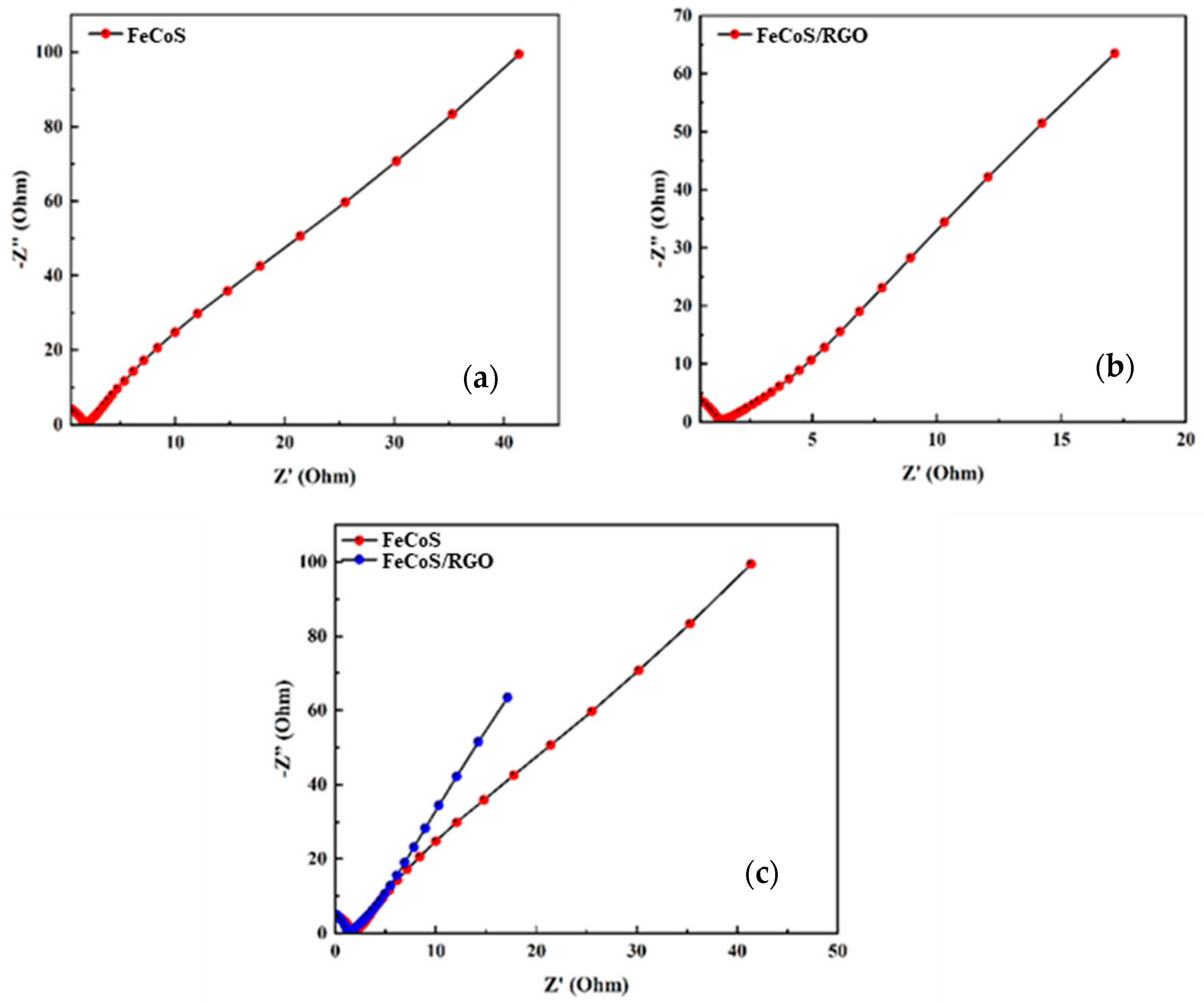
3.7. Nyquist Plots
Electrochemical impedance spectroscopy (EIS) was conducted on both Fe
0.8Co
0.2S and Fe
0.8Co
0.2S/RGO composites, see
Figure 8a and
Figure 8b, respectively, in order to evaluate their charge storage behavior and interfacial kinetics. The Nyquist plot reveals enhanced EDLC behavior upon the incorporation of RGO, as evidenced by the upward shift in the low-frequency tail of the curve. Furthermore, the Fe
0.8Co
0.2S/RGO composite exhibits a significantly reduced charge transfer resistance compared to the pristine material, as indicated by the smaller semicircle diameter in the high-frequency region as shown in
Figure 8c. This enhanced behavior implies that RGO improves conductivity and provides a channel for quicker charge transfer, placing the composite in the running for high-performance energy storage applications.
4. Conclusions
In this work, Fe0.8Co0.2S and Fe0.8Co0.2S/rGO composites were successfully synthesized, and their electrochemical performance as supercapacitor materials was assessed. The addition of rGO to the Fe0.8Co0.2S matrix greatly improved the specific capacitance, as indicated by a >283% increase compared to pure Fe0.8Co0.2S. Galvanostatic charge–discharge tests also verified this improvement, indicating a remarkable increase in discharge time. Characterization methods, such as XRD, SEM, and EDS, confirmed the successful formation of the composite and indicated the synergistic effect of rGO in enhancing the material’s structural and electrochemical properties. These findings illustrate the potential of Fe0.8Co0.2S/rGO composites as effective electrode materials for high-performance supercapacitors, emphasizing the significance of rGO integration for increased energy storage capacity. Additional optimization of the composition and the morphology of the composite may provide enhanced electrochemical performance, enabling advanced energy storage devices.
Author Contributions
This study was designed, planned and supervised by M.A.M. The experimental work was performed, and initial draft was written by M.T. All authors have read and agreed to the published version of the manuscript.
Funding
We thank the GIK Institute and HEC (NRPU project no. 16196) for their financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data will be provided by the first author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Winchester, A.; Ghosh, S.; Feng, S.; Elias, A.L.; Mallouk, T.; Terrones, M.; Talapatra, S. Electrochemical Characterization of Liquid Phase Exfoliated Two-Dimensional Layers of Molybdenum Disulfide. ACS Appl. Mater. Interfaces 2014, 6, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Marwat, M.A.; Khan, M.F.; Adam, K.M.; Din, Z.U.; Karim, M.R.A.; Khan, S. Ti3SiC2-coupled NiCoMn LDH nanocomposites as positive electrode for high performance supercapacitors. J. Alloys Compd. 2023, 956, 170229. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Bundaleska, N.; Abrashev, M.; Kissovski, J.; Bundaleski, N.; Teodoro, O.M.N.D.; de Ferro, A.M.; Silva, R.P.; Dias, A.; Felizardo, E.; et al. Free-standing graphene-carbon as negative and FeCoS as positive electrode for asymmetric supercapacitor. J. Energy Storage 2022, 50, 104637. [Google Scholar] [CrossRef]
- Shinde, S.K.; Jalak, M.B.; Kim, S.Y.; Yadav, H.M.; Ghodake, G.S.; Kadam, A.A.; Kim, D.Y. Effect of Mn doping on the chemical synthesis of interconnected nanoflakes-like CoS thin films for high performance supercapacitor applications. Ceram. Int. 2018, 44, 23102–23108. [Google Scholar] [CrossRef]
- Jansi Rani, B.; Pradeepa, S.S.; Hasan, Z.M.; Ravi, G.; Yuvakkumar, R.; Hong, S.I. Supercapacitor and OER activity of transition metal (Mo, Co, Cu) sulphides. J. Phys. Chem. Solids 2020, 138, 109240. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Srivastava, R.; Mageto, T.; Chaudhari, M.; Kumar, A.; Sultana, J.; Mishra, S.R.; Perez, F.; Gupta, R.K. Bimetallic Co–Fe sulfide and phosphide as efficient electrode materials for overall water splitting and supercapacitor. Discov. Nano 2023, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Forouzandeh, P.; Kumaravel, V.; Pillai, S.C. Electrode Materials for Supercapacitors: A Review of Recent Advances. Catalysts 2020, 10, 969. [Google Scholar] [CrossRef]
- Xu, T.; Li, G.; Yang, X.; Guo, Z.; Zhao, L. Design of the seamless integrated C@NiMn-OH-Ni3S2/Ni foam advanced electrode for supercapacitors. Chem. Eng. J. 2019, 362, 783–793. [Google Scholar] [CrossRef]
- Raza, M.A.; Yousaf, M.I.; Akram, S.; Siddique, A.; Ashraf, M.; Fu, D.J. High-performance supercapacitor based on NiCo2S4/g-C3N4/polyaniline deposited on carbon cloth for structural stability. Synth. Met. 2024, 301, 117534. [Google Scholar] [CrossRef]
- Karimi, A.; Kazeminezhad, I.; Naderi, L.; Shahrokhian, S. Construction of a Ternary Nanocomposite, Polypyrrole/Fe–Co Sulfide-Reduced Graphene Oxide/Nickel Foam, as a Novel Binder-Free Electrode for High-Performance Asymmetric Supercapacitors. J. Phys. Chem. C 2020, 124, 4393–4407. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).