Performance Assessment of Fe0.5Cu0.5S/rGO Hybrid Composite as Potential Material for Advanced Energy Storage Applications †
Abstract
1. Introduction
2. Experimental
2.1. Materials
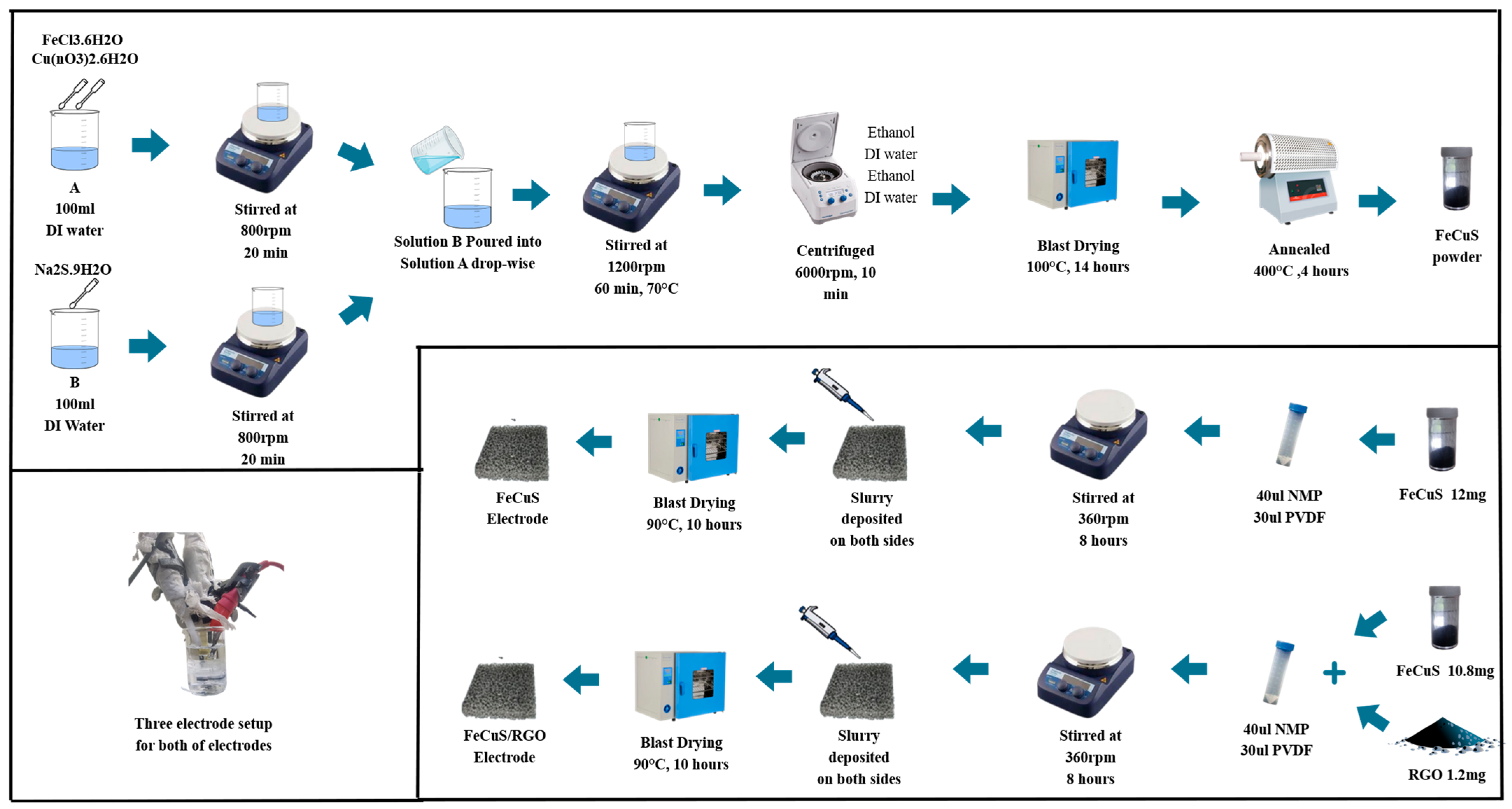
2.2. Synthesis
3. Results
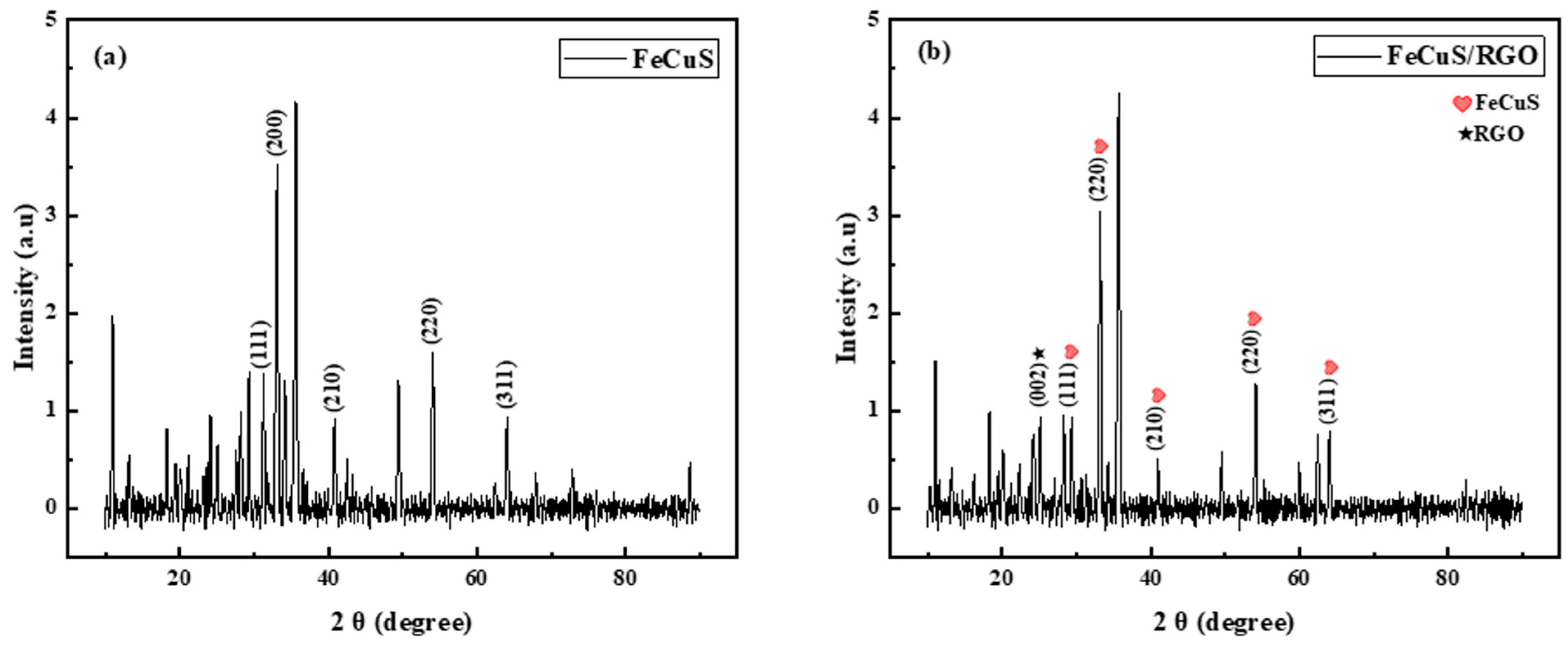
3.1. X-Ray Diffraction
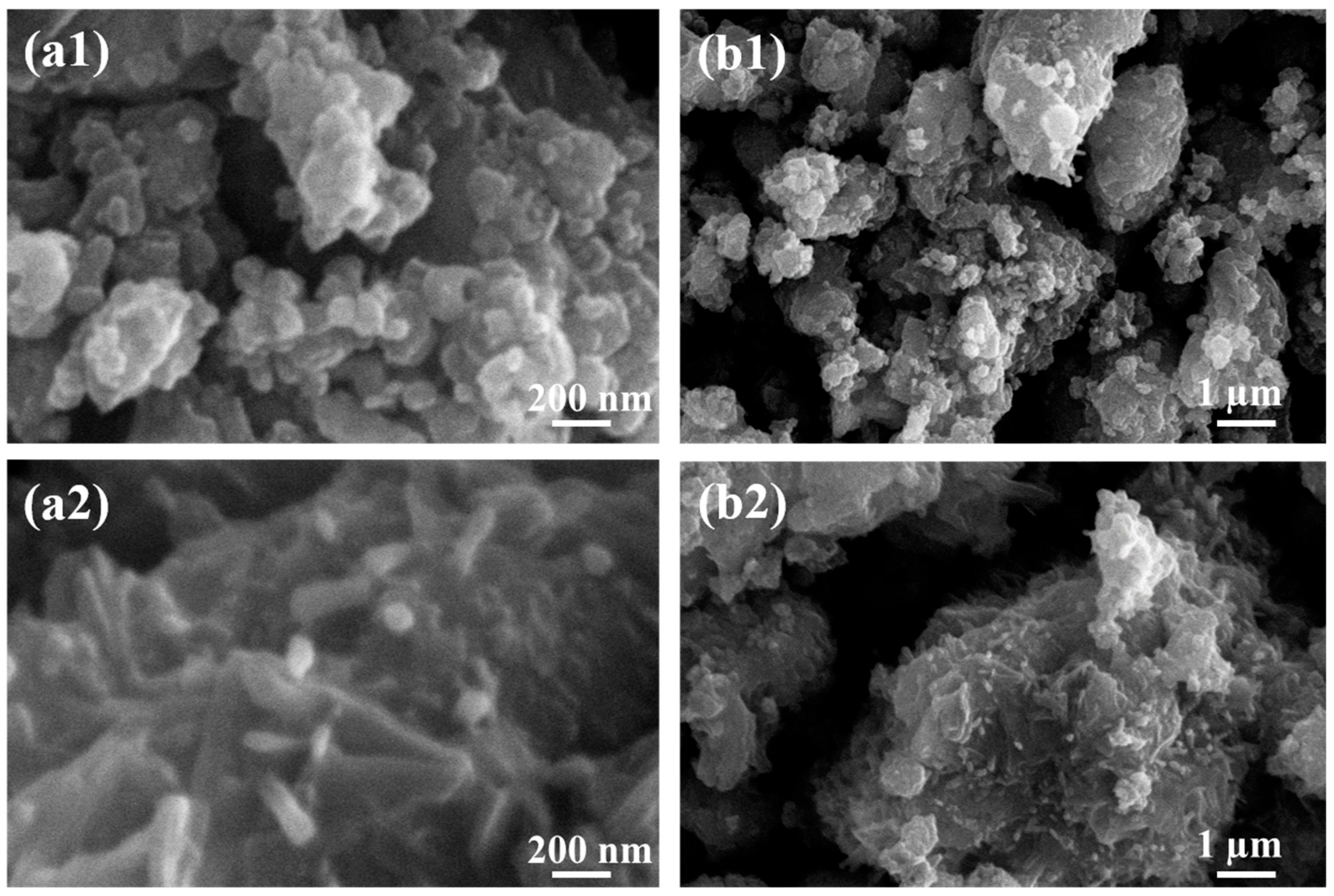
3.2. Scanning Electron Microscopy
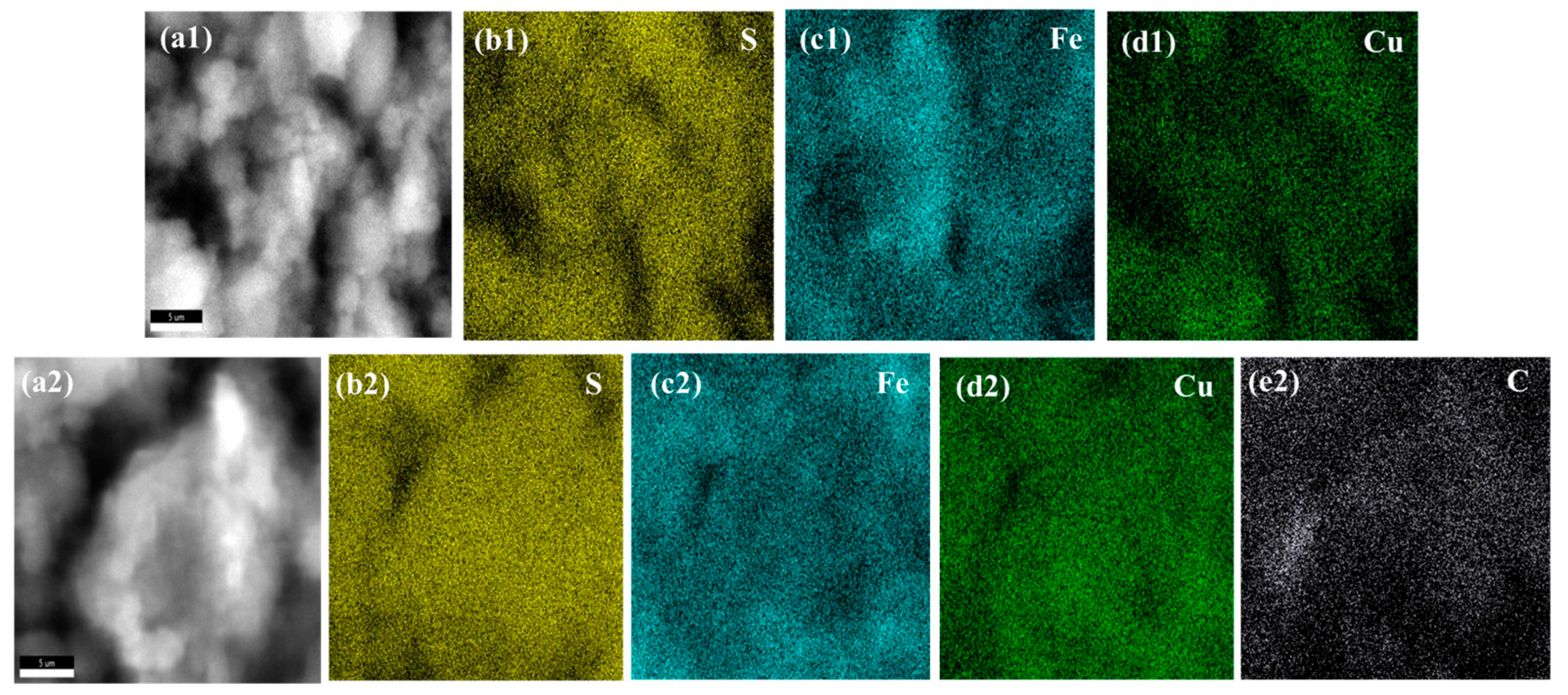
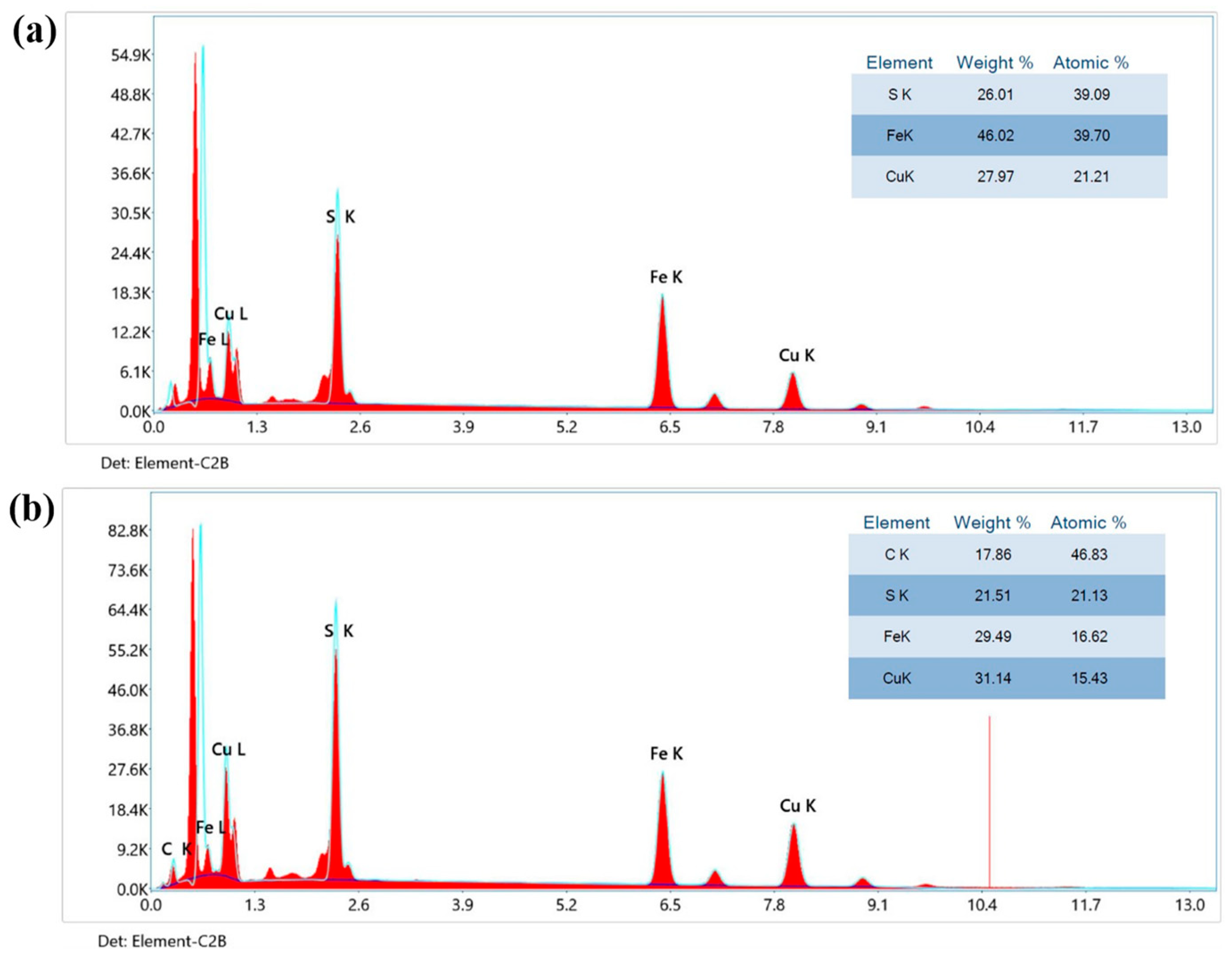
3.3. Energy Dispersive Spectroscopy
3.4. Cyclic Voltammetry (CV)
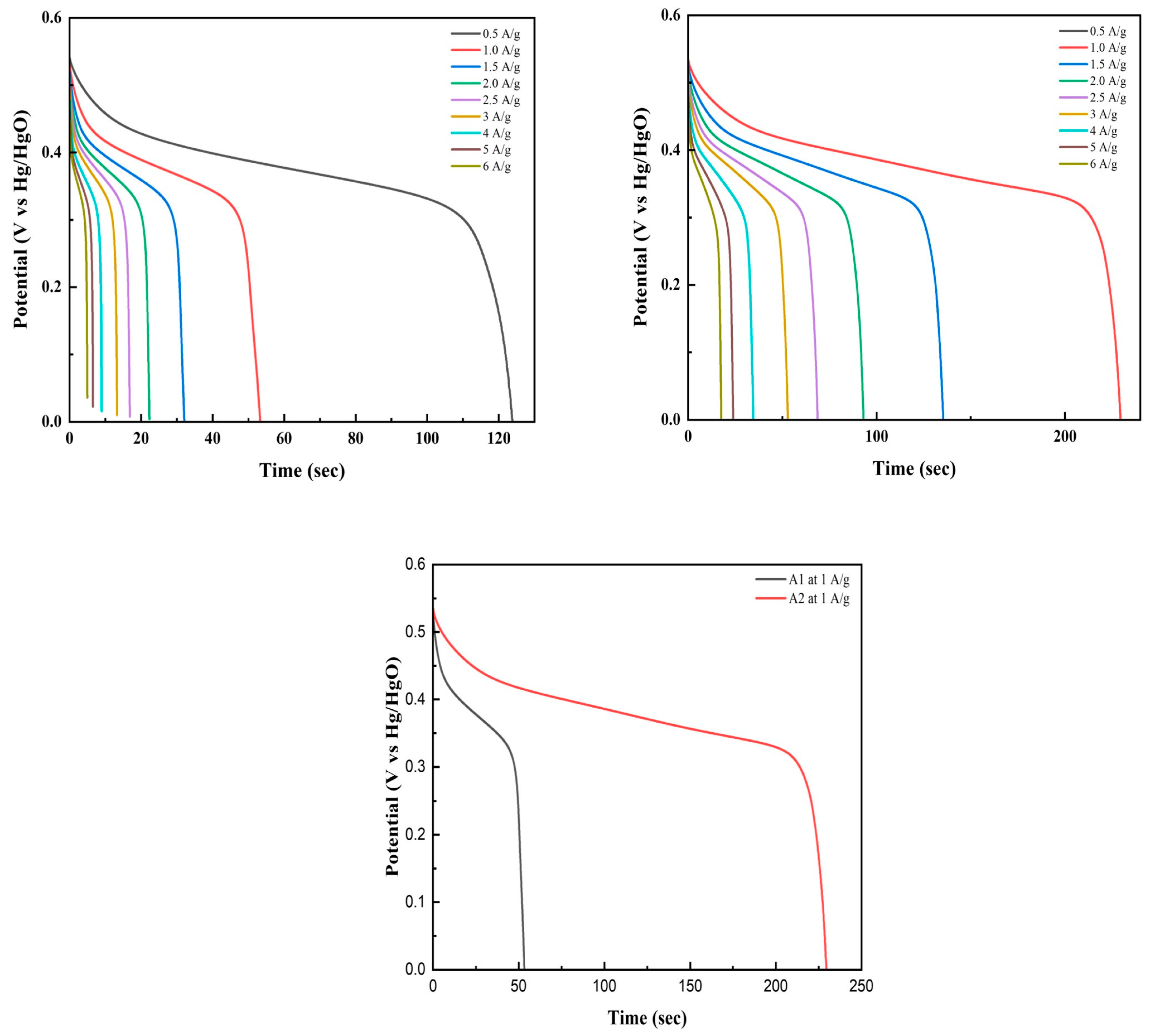
3.5. Galvanostatic Charge Discharge (GCD)
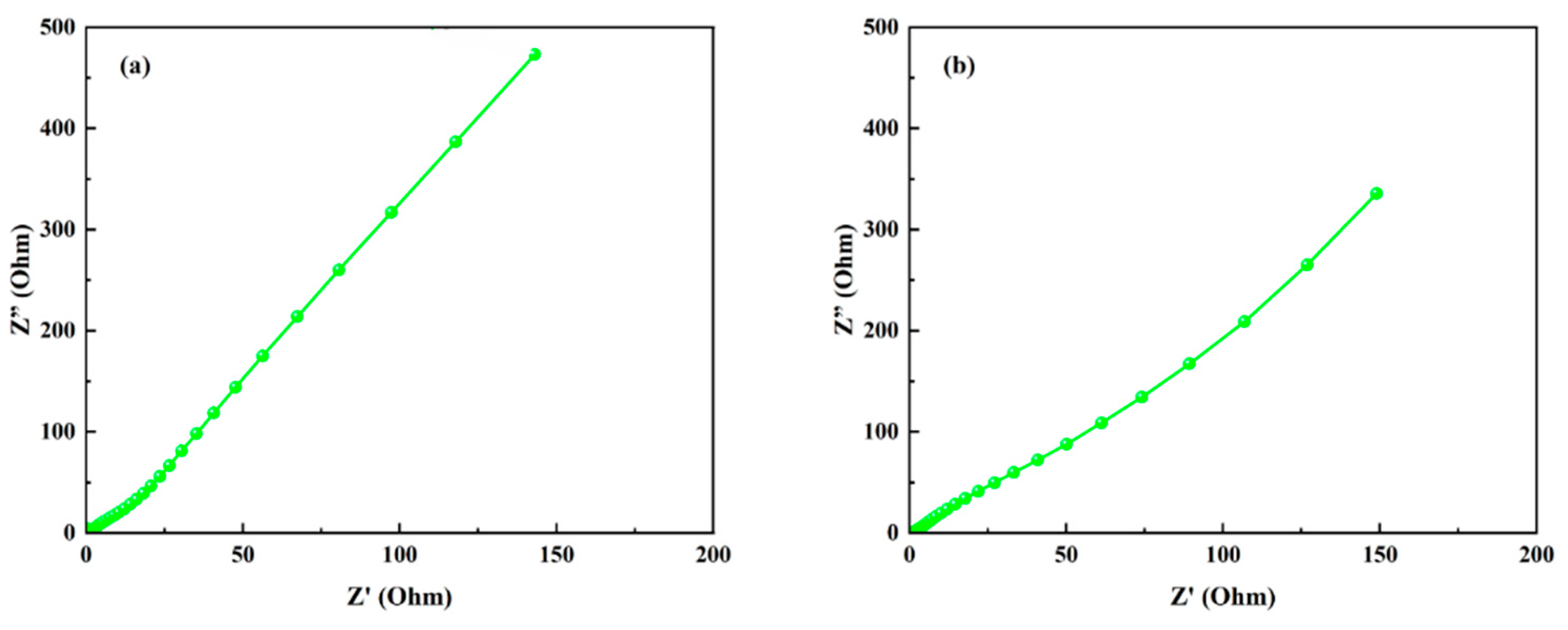
3.6. Nyquist Plots
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shuja, A.; Khan, H.R.; Murtaza, I.; Ashraf, S.; Abid, Y.; Farid, F.; Sajid, F. Supercapacitors for energy storage applications: Materials, devices and future directions: A comprehensive review. J. Alloys Compd. 2024, 1009, 176924. [Google Scholar] [CrossRef]
- Zhang, S.; Wen, J.; Tian, Y.; Ning, H.; Yang, H.; Shu, Q.; Hu, N. Flexible supercapacitors based on in-situ synthesis of composite nickel manganite@reduced graphene oxide nanosheets cathode: An integration of high mechanical flexibility and energy storage. J. Alloys Compd. 2024, 1009, 176873. [Google Scholar] [CrossRef]
- Marwat, M.A.; Khan, M.F.; Humayun, M.; Ali, S.; Karim, M.R.A.; Shah, S.S.; Bououdina, M.; Din, Z.U.; Adam, K.M.; Abdullah, S.M. Novel NiCoMn MOFs/Ag citrate nanocomposites for high-performance asymmetric supercapacitor applications. Electrochim. Acta 2025, 511, 145373. [Google Scholar] [CrossRef]
- Marwat, M.A.; Ishfaq, S.; Adam, K.M.; Tahir, B.; Shaikh, M.H.; Khan, M.F.; Karim, M.R.A.; Din, Z.U.; Abdullah, S.; Ghazanfar, E. Enhancing supercapacitor performance of Ni–Co–Mn metal–organic frameworks by compositing it with polyaniline and reduced graphene oxide. RSC Adv. 2024, 14, 2102–2115. [Google Scholar] [CrossRef] [PubMed]
- Hamayun, U.; Marwat, M.A.; Abdullah, S.M.; Ullah, R.; Humayun, M.; Bououdina, M.; Karim, M.R.A.; Khan, M.Z.; Hanif, M.B. Synergistic integration of Ag@Fe0.67Cu0.22Co0.11S core-shell nanostructures and SWCNTs for improved supercapacitor performance. J. Alloys Compd. 2025, 1012, 178422. [Google Scholar] [CrossRef]
- Singhal, R.; Thorne, D.; Chaudhary, M.; Kumar, A.; Bhardwaj, S.; Abazi, R.; Colon, A.; Gupta, R.K.; Scanley, J.; Broadbridge, C.C.; et al. Studies of reduced graphene oxide (rGO)/CuS nanocomposite for supercapacitor applications. AIP Adv. 2023, 13, 125011. [Google Scholar] [CrossRef]
- Venkateshalu, S.; Kumar, P.G.; Kollu, P.; Jeong, S.K.; Grace, A.N. Solvothermal synthesis and electrochemical properties of phase pure pyrite FeS2 for supercapacitor applications. Electrochim. Acta 2018, 290, 378–389. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, H.; Guo, P.; Li, J.; Zhu, H.; Fan, X.; Huang, L.; Sun, J.; Wang, Y. Application of copper-sulfur compound electrode materials in supercapacitors. Molecules 2024, 29, 977. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Gopi, C.V.V.M.; Kim, H.J. Enhanced electrochemical capacitance of polyimidazole coated covellite CuS dispersed CNT composite materials for application in supercapacitors. Dalton Trans. 2016, 45, 12362–12371. [Google Scholar] [CrossRef] [PubMed]
- Ranjani, P.R.; Anjana, P.M.; Rakhi, R.B. Solvothermal synthesis of CuFeS2 nanoflakes as a promising electrode material for supercapacitors. J. Energy Storage 2021, 33, 102063. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arif, A.; Murtaza, H.; Marwat, M.A.; Karim, M.R.A.; Ijaz, S. Performance Assessment of Fe0.5Cu0.5S/rGO Hybrid Composite as Potential Material for Advanced Energy Storage Applications. Mater. Proc. 2025, 23, 14. https://doi.org/10.3390/materproc2025023014
Arif A, Murtaza H, Marwat MA, Karim MRA, Ijaz S. Performance Assessment of Fe0.5Cu0.5S/rGO Hybrid Composite as Potential Material for Advanced Energy Storage Applications. Materials Proceedings. 2025; 23(1):14. https://doi.org/10.3390/materproc2025023014
Chicago/Turabian StyleArif, Anusha, Hasnain Murtaza, Mohsin Ali Marwat, Muhammad Ramzan Abdul Karim, and Shariq Ijaz. 2025. "Performance Assessment of Fe0.5Cu0.5S/rGO Hybrid Composite as Potential Material for Advanced Energy Storage Applications" Materials Proceedings 23, no. 1: 14. https://doi.org/10.3390/materproc2025023014
APA StyleArif, A., Murtaza, H., Marwat, M. A., Karim, M. R. A., & Ijaz, S. (2025). Performance Assessment of Fe0.5Cu0.5S/rGO Hybrid Composite as Potential Material for Advanced Energy Storage Applications. Materials Proceedings, 23(1), 14. https://doi.org/10.3390/materproc2025023014




