Abstract
A gradual surge in the population on Earth has increased the demand for food. Various synthetic materials have been used for food packaging for a long time. These materials are contaminating our environment and disrupting human life and that of other species. This study was conducted to minimize the impact of the pollution caused by using plastics for conventional packaging. A green approach to synthesizing packaging material that prevents food contamination with improved mechanical properties was adopted. Firstly, extracts were obtained from grapes and tomatoes and dissolved into four different solvents, i.e., de-ionized water, dichloromethane, ethyl acetate, and n-hexane. Three different extract solutions were made in de-ionized water, varying the fraction of the extract and de-ionized water. The extracts were then tested for the presence of various phytochemicals. The solutions were then combined with cyclodextrin, starch, alginate, and polyvinyl alcohol, all of which are biodegradable, non-cytotoxic, and pocket-friendly. Calcium chloride was also added because it acts as a firming agent and a desiccant. This resulted in the formation of a total of six membranes with four different solvents. These membranes had varying degrees of biodegradability and antibacterial properties. Various phytochemicals, such as saponins, flavonoids, terpenoids, carotenoids, tannins, phenols, and steroids, were found in the fruit extracts. These phytochemicals act as anti-microbial and anti-fungal agents. Out of the six different membranes that were synthesized, the membrane with a 7:3 composition of crude extract to de-ionized water showed the best results for use as a packaging material, as it showed the best antibacterial properties and good reported biodegradability. The FTIR results for this membrane showed bands at around 3500 cm−1, indicating the presence of -OH and -NH functionality since these bands overlap and cannot be distinguished at this position. The shoulder band indicates the presence of carboxylic acid -OH. Integrating biopolymers with fruit extracts enhances the nutritional value of food and provides an eco-friendly and cost-effective approach to packaging material synthesis. The synthesized membranes are cost-effective as they contain fruit extracts from grapes and tomatoes; starch; and cyclodextrin. The extracts obtained from the fruits were inexpensive, as 300 mL of extract cost around 300 Rs. The synthesized membranes had functional advantages such as biodegradability and providing an enhanced shelf life to food products. Hence, they reduce the losses caused by food spoilage. Another driver of their cost effectiveness is that they can reduce waste disposal costs on the one hand and environmental pollution on the other hand.
1. Introduction
The “Plastic Age” has stimulated the demand for ecologically suitable alternatives in response to the ecological catastrophe caused by plastics. To fulfill sustainability objectives, researchers are focusing on bio-based polymers and biocomposites that use renewable resources, such as tailored starch-based plastics [1]. Starch-based edible films are sustainable packaging supplies that exploit the unique properties of starch to create thin, flexible food packaging films. They feature biodegradable barriers and can be employed in culinary applications. They are produced using extrusion, solvent casting, or compression molding [2]. Starch, a polysaccharide present in maize, wheat, rice, potatoes, and other plant-based foods, is a biodegradable and renewable polymer with several uses. Its distinctive composition and biodegradability make it an appealing option for eco-friendly packaging materials. Starch-based packaging is an environmentally benign alternative to polymers derived from fossil fuels since it lowers environmental contamination and the accumulation of non-biodegradable waste, especially for single-use applications [3]. Conventional packaging protects food against spoilage and infection. The food packaging business is becoming more concerned with intelligent packaging, which includes sensing, recording, and detecting capabilities. Intelligent packaging solutions improve traceability, optimize logistics, and decrease waste by monitoring food conditions and providing quality data. They account for around 5% of the industry’s value, and the demand is increasing rapidly [4]. Plastic’s fluidity, moldability, heat salability, simplicity of printing, and integration into manufacturing processes make it a desirable material for food packaging. Beyond unfavorable customer perceptions the short lifespan, enormous volume, and poor recyclability of plastic all contribute to major solid waste issues, particularly in aquatic ecosystems [5]. Despite being used in food packaging, non-biodegradable polymers such as polyethylene, propylene, and polystyrene disintegrate at a slower pace than biodegradable composites, taking years to break down in the environment [6]. Biodegradable materials, such as multilayer and composite constructions, are used for food packaging since they are more environmentally friendly, have longer shelf lives, and provide barrier protection. However, due to complicated production methods and high raw material prices, biodegradable packaging materials are more expensive [7]. This work filled a research gap by synthesizing food packaging material from easily available and low-cost sources that were non-cytotoxic and extended food’s shelf life. Previously, alginate-films had been synthesized for food packaging. Biopolymers such as cyclodextrin and starch were combined with tomato and grape extracts to create membranes using a membrane casting method [8]. Alginate and polyvinyl alcohol were utilized as the cross-linking agents. First, the extracts were evaluated for the presence of different phytochemicals. These compounds have antioxidant and anti-inflammatory properties. Membranes were made using a variety of solvents, including distilled water, dichloromethane, ethyl acetate, and n-hexane. These membranes were then evaluated for their antibacterial activity, biodegradability, and stability in extending the shelf lives of food products. The membrane with a 7:3 ratio of crude extract to water produced the best results and thus could be utilized as food packaging material.
The current study was carried out to improve the strength, flexibility, and durability of food packaging applications; to effectively inhibit microbial development and prevent food from deteriorating; to promote the steady and progressive release of beneficial molecules to maintain food’s freshness and quality over time; and to reduce environmental pollution by offering environmentally friendly alternatives to conventional plastic packaging.
Food packaging frequently used to be made of synthetic plastics, but because of their associated disadvantages, such as the possibility of cancer, the accumulation of solid waste, and environmental pollution, we have created biodegradable membranes that are derived from natural sources. Because of their enhanced environmental friendliness, longer shelf lives, natural antibacterial activity, sustainability, and reduced food spoilage, these bio-based packaging materials are excellent options for environmentally friendly food packaging.
2. Materials and Methods
Methodology
The following materials (Table 1) and equipments were used for this study: cyclodextrin (Sigma-Aldrich, St. Louis, MO, USA), starch (Sigma-Aldrich, St. Louis, MO, USA), n-hexane (95%, Sigma-Aldrich, St. Louis, MO, USA), ethyl acetate (99%, Sigma-Aldrich, St. Louis, MO, USA), dichloromethane (Sigma-Aldrich, St. Louis, MO, USA), tomatoes and grapes (a local market at Karachi Company, Islamabad, Pakistan), alginate (87.6 ± 0.2%, Merck, Darmstadt, Germany), polyvinyl alcohol (Merck, Darmstadt, Germany), calcium chloride (>96%, Sigma-Aldrich, St. Louis, MO, USA), ferric chloride, (93%, Sigma-Aldrich, St. Louis, MO, USA), chloroform (DAEJUNG, Siheung-si, Gyeonggi-do, South Korea), ethanol (99.5%, DAEJUNG, Siheung-si, Gyeonggi-do, South Korea), sulfuric acid (37%, Riedel-de Haën, Seelze, Germany), hydrochloric acid (37%, Riedel-de Haën, Seelze, Germany), sodium hydroxide (≥98%, pellets, Sigma-Aldrich, St. Louis, MO, USA), a shaker (Kalstein, Paris, France), and a hotplate (Kalstein, Paris, France). The method for the synthesis followed is as follow (Figure 1).

Table 1.
Composition of the membranes.
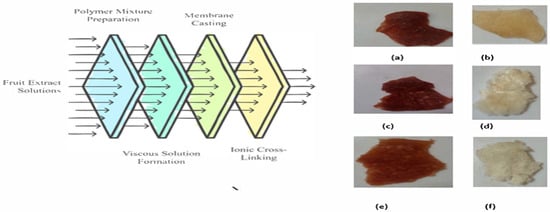
Figure 1.
Schematics of membrane synthesis. (a) The 7:3 membrane, (b) the dicholoromethane membrane, (c) the 3:7 membrane, (d) the ethyl acetate membrane, (e) the 5:5 membrane, and (f) the n-hexane membrane.
To check for saponins in the fruit extract, 2 mL of the extract was pipetted out. Then, 6 mL of distilled water was added, and it was shaken well on a shaker apparatus. To evaluate tannins, 2 mL of the extract was pipetted out, and then, 3 mL of distilled water was added. It was shaken well on the shaker apparatus, and drops of ferric chloride were added [9]. To test for the presence of flavonoids, NaOH was added to the test tube containing the fruit extract. To examine terpenoid, 2 mL of the extract was taken and then treated with 2 mL of chloroform. Afterwards, 3 mL of concentrated H2SO4 was added [10]. To test for carotenoids, 2 mL of the extracts were put into a test tube, and then 10 mL of chloroform was added. It was then shaken well. This mixture was filtered out and treated with 85% sulfuric acid. To test for phenols, 2 mL of extract was pipetted out into a test tube, and 10% aqueous FeCl3 solution was added, as per literature. To assess steroids, 2 mL of the extract was pipetted out, and then 6 drops of concentrated H2SO4 were added slowly from the side wall of the test tube.
3. Results and Discussion
3.1. The Phytochemical Analysis
Table 2 below shows the presence of various phytochemicals in the food extracts after testing. These phytochemicals are essential for health, as they exhibit antioxidant properties, antibacterial properties, and anti-fungal properties.

Table 2.
A table on the presence of various phytochemicals.
3.2. Antibacterial Activity
The synthesized membranes were tested for their antimicrobial activity. For this purpose, a disc diffusion method was utilized. As shown in Figure 2, on a layer of Mueller–Hinton agar, the non-pathogenic bacterial strain E. coli was grown, and the membrane solution was casted onto the discs and placed on the colony. For the positive control, 10 mg of the antibiotic Norfloxacin was used. The effects on growth inhibition were observed and recorded after a period of 24 h and expressed as follows.
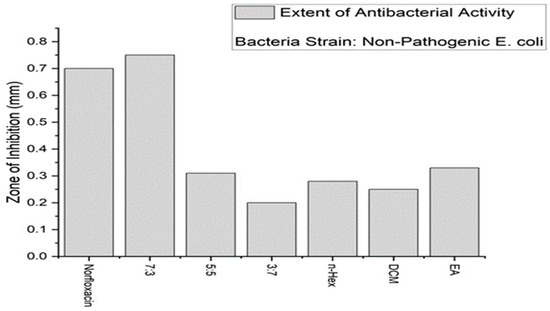
Figure 2.
A bar chart of the antibacterial activity.
From the bar chart, it is concluded that the membrane with a 7:3 composition of the crude extract and water showed the best antibacterial results. This means that if the food is packed in it, there will be negligible or no bacterial growth. The food will be prevented from spoilage. The shelf-life of the food will be increased.
In Figure 3 below, the size of the zone of inhibition caused by the standard is 0.70 mm. The membrane with a 7:3 ratio has the biggest size of 0.75 mm as compared to that with the standard.

Figure 3.
The antibacterial activity on a layer of Mueller–Hinton agar. A bacterial strain of E. coli was grown onto the layer, and the membrane solution was cast onto discs.
E. coli strain was resistant to pomegranate extract [11].
3.3. Biodegradability
To determine biodegradability, the soil burial method was utilized. The initial masses of all six samples were calculated. Afterwards, the samples were buried 15 cm deep in the soil, and the changes in their masses and physical characteristics were observed every 15 days. The overall mass changes after a period of 7 days are expressed as follows in Figure 4:
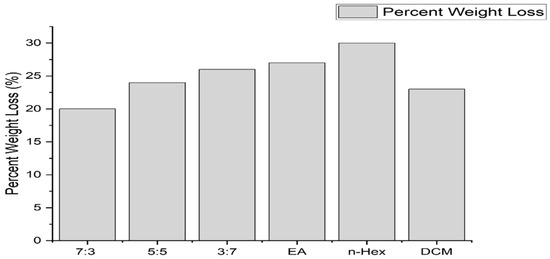
Figure 4.
A bar chart of the biodegradability.
As shown in Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10, all of the samples showed considerable mass losses, and thus, we can infer that the composite material produced was indeed biodegradable. The material is considerably resistant to degradation in the open air at room temperature due to its antimicrobial activity. However, after dumping it in the soil, it slowly started to degrade over time; the n-hexane sample was that which degraded the least, and the 7:3 membrane degraded the most. The membrane with a 7:3 fraction showed considerable weight loss over time. Thus, it is highly biodegradable. Hence, it can reduce the cost of disposal and reduce the contamination of the environment with plastic. Meanwhile, bioplastics degraded into biomass in 6–12 weeks [12].
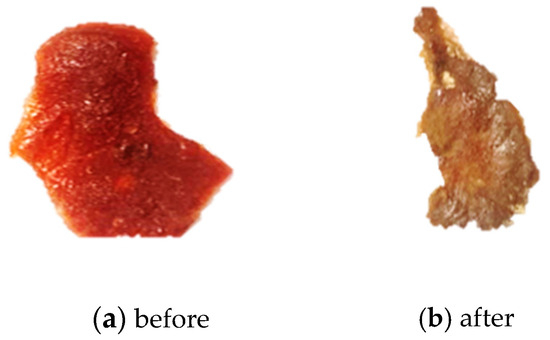
Figure 5.
(a) An image of the 7:3 membrane before burial in soil. (b) An image of the 7:3 membrane recovered from the soil.

Figure 6.
(a) An image of the 3:7 membrane before burial in soil. (b) An image of the 3:7 membrane recovered from the soil.
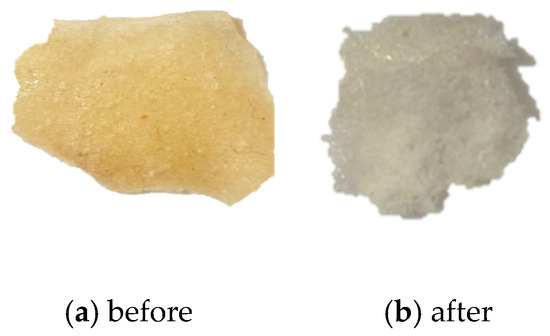
Figure 7.
(a) An image of the DCM membrane before burial in soil. (b) An image of the DCM membrane recovered from the soil.
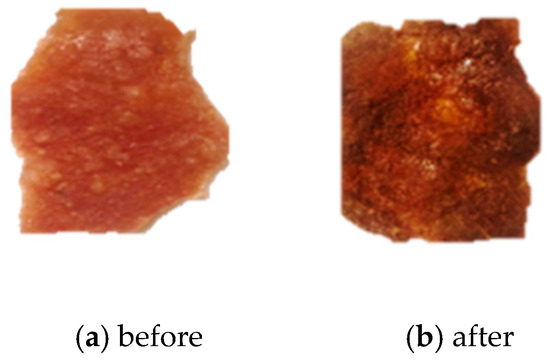
Figure 8.
(a) An image of the 5:5 membrane before burial in soil. (b) An image of the 5:5 membrane recovered from soil.
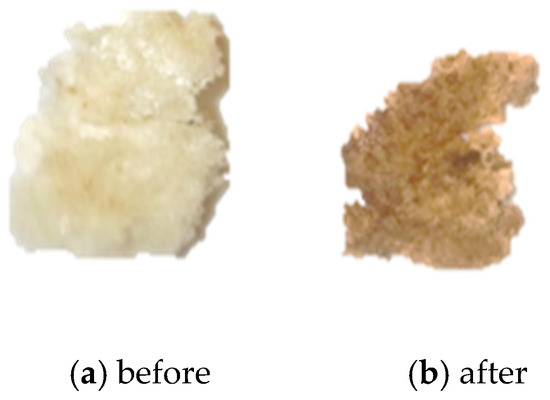
Figure 9.
(a) An image of the ethyl acetate membrane before burial in soil. (b) An image of the ethyl acetate membrane recovered from the soil.
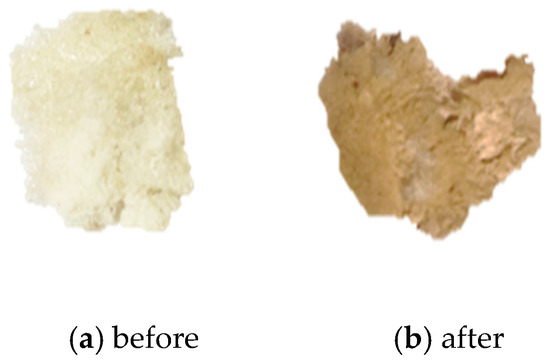
Figure 10.
(a) An image of the n-hexane membrane before burial in the soil. (b) An image of the n-hexane membrane after recovery from the soil.
3.4. Fourier Transform Infrared Spectroscopy
The FTIR (using a Bruker Alpha Platinum ATR, Ettlingen, Germany) analysis as given was performed using a range of 4000−500 cm−1. The FTIR spectra of the membrane with a 7:3 fraction and the fruit extract are given below.
There is a strong band at 3400 cm−1 which corresponds to the presence of O−H bond or the N-H bond stretching. This suggests the presence of polyphenols in the membrane. There is a shoulder membrane at 2900 cm−1 this corresponds to C−H bond stretching. This shows the presence of a long polymeric chain of aliphatic hydrocarbons. Next, there is a band at 1700 cm−1 due to the carbonyl group, indicating the presence of alginate and starch. While, the band at 1600−1500 cm−1 suggests the presence of aromatic C=C bond or N−H bond bending in the amide group (as shown in Figure 11).
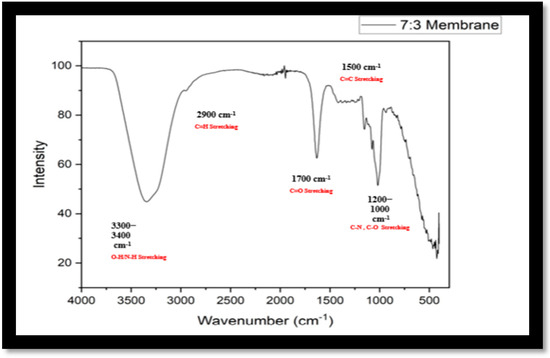
Figure 11.
FTIR spectrum of membrane with 7:3 fraction.
There is a band at 3300−3400 cm−1; this is due to the presence of the O-H bond stretching. The band is also broad due to the presence of hydrogen bonding, representing the presence of flavonoids and other polyphenolic groups. The next band is at 2900 cm−1, which suggests the presence of an aliphatic C−H bond. The band around 1700 cm−1 suggests that carbonyl groups in the form of plant secondary metabolites are present. The band within the range of 1600−1400 cm−1 reflects C=C stretching in the form of an aromatic ring. While the band between 1200 and 1000 cm−1 indicates C−O stretching. This C−O is the characteristic functional group of alcohols, ethers, and phenols (as shown in Figure 12).
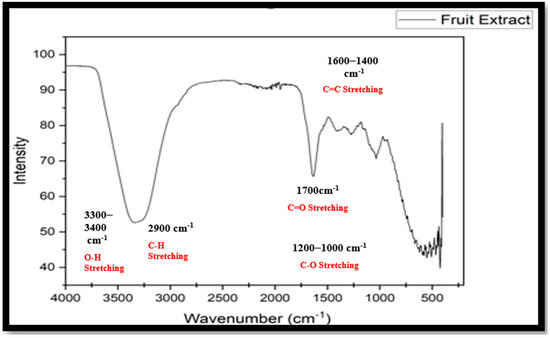
Figure 12.
FTIR spectrum of fruit extract.
4. Conclusions
Conventional plastic carries enormous risks of environmental pollution and toxicity. Because of these concerns, the search for alternatives is growing, and suitable composites of natural polymers emerge as a viable option. For this purpose, this research was carried out to identify perfect candidates that could provide a cost-effective solution and that also possessed the inherent capabilities of plant secondary metabolites and biodegradability and caused no threat to the environment. Fruit extracts from tomatoes and grapes were mixed into various solvents, and membranes using cyclodextrin, starch, and alginate were made. A total of six membranes were synthesized and tested. The membrane with a 7:3 fraction showed the best results for use as food packaging material, as this membrane had the largest-sized zone of inhibition, which was 0.75 mm, as compared with that with the standard. When this membrane, along with the other membranes, was tested for biodegradability, this membrane experienced the greatest weight loss when it was buried in the soil. Hence, biodegradable membranes from natural sources are being used for food packaging due to their environmental friendliness, longer shelf life, antibacterial activity, sustainability, and reduced spoilage compared to these properties in synthetic plastics. The FTIR spectra of the membrane with the 7:3 fraction and the fruit extracts suggested the presence of essential chemicals such as polyphenols, flavonoids, sugars, carbohydrates, and alkaloids. These not only avoid food contamination but are also a source of anti-cancer and anti-inflammatory agents. The phytochemical analysis detected the presence of saponins, tannins, terpenoids, flavonoids, carotenoids, and phenols in the fruit extract. These chemicals increase the shelf-life of the food by acting as anti-microbial and anti-fungal agents. They preserve the nutritional value of the food and also promote health effects by slowly migrating into the food.
Author Contributions
Conceptualization, S.A.K.; methodology, K.A.K. and T.A.; software, M.H.L. and K.K.; validation, T.A. and K.A.K.; formal analysis, M.W.; investigation, T.A.; resources, H.A. and S.A.K.; data curation, A.T.J.; writing—original draft preparation, K.A.K. and K.K.; writing—review and editing, A.Y.; visualization, A.T.J.; supervision, S.A.K.; project administration, H.A.; funding acquisition, S.A.K. and H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akhrib, S.; Djellali, S.; Haddaoui, N.; Karimian, D.; Carraro, M. Biocomposites and poly (lactic acid) in active packaging: A review of current research and future directions. Polymers 2024, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Ma, H.; Xie, Q.; Guo, J.; Liu, G.; Zhang, B.; Li, X.; Zhang, Q.; Cao, Q.; Li, X. Developing active and intelligent biodegradable packaging from food waste and byproducts: A review of sources, properties, film production methods, and their application in food preservation. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13334. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Palanisamy, C.P.; Srinivasan, G.P.; Panagal, M.; Kumar, S.S.D.; Mironescu, M. A comprehensive review on starch-based sustainable edible films loaded with bioactive components for food packaging. Int. J. Biol. Macromol. 2024, 274, 133332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, K.; Zhang, M.; Xu, T.; Du, H.; Pang, B.; Si, C. Sustainable polysaccharide-based materials for intelligent packaging. Carbohydr. Polym. 2023, 313, 120851. [Google Scholar] [CrossRef] [PubMed]
- Kan, M.; Miller, S.A. Environmental impacts of plastic packaging of food products. Resour. Conserv. Recycl. 2022, 180, 106156. [Google Scholar] [CrossRef]
- Arman Alim, A.A.; Mohammad Shirajuddin, S.S.; Anuar, F.H. A review of nonbiodegradable and biodegradable composites for food packaging application. J. Chem. 2022, 2022, 7670819. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, W.; Zhu, W.; McClements, D.J.; Liu, X.; Liu, F. A review of multilayer and composite films and coatings for active biodegradable packaging. NPJ Sci. Food 2022, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Janik, W.; Jakubski, Ł.; Kudła, S.; Dudek, G. Modified polysaccharides for food packaging applications: A review. Int. J. Biol. Macromol. 2024, 258, 128916. [Google Scholar] [CrossRef] [PubMed]
- Harbone, J. Experimental Methods in Analytical Chemistry; Chapman and Hall: New York, NY, USA, 1973; pp. 140–145. [Google Scholar]
- Maheshwaran, L.; Nadarajah, L.; Senadeera, S.; Ranaweera, C.; Chandana, A.; Pathirana, R. Phytochemical Testing Methodologies and Principles for Preliminary Screening/Qualitative Testing. Asian Plant Res. J. 2024, 12, 11–38. [Google Scholar] [CrossRef]
- Dilucia, F.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Sustainable use of fruit and vegetable by-products to enhance food packaging performance. Foods 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Rocculi, P.; Romani, S.; Dalla Rosa, M. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).