Melting Boundaries: How Heat Transforms Recycled Bottles into Chemical Time Bombs †
Abstract
1. Introduction
2. Methodology
2.1. Sample Preparation and PAEs Extraction
2.2. Thermal Analysis for the Plastic
3. Results
3.1. Leaching Result
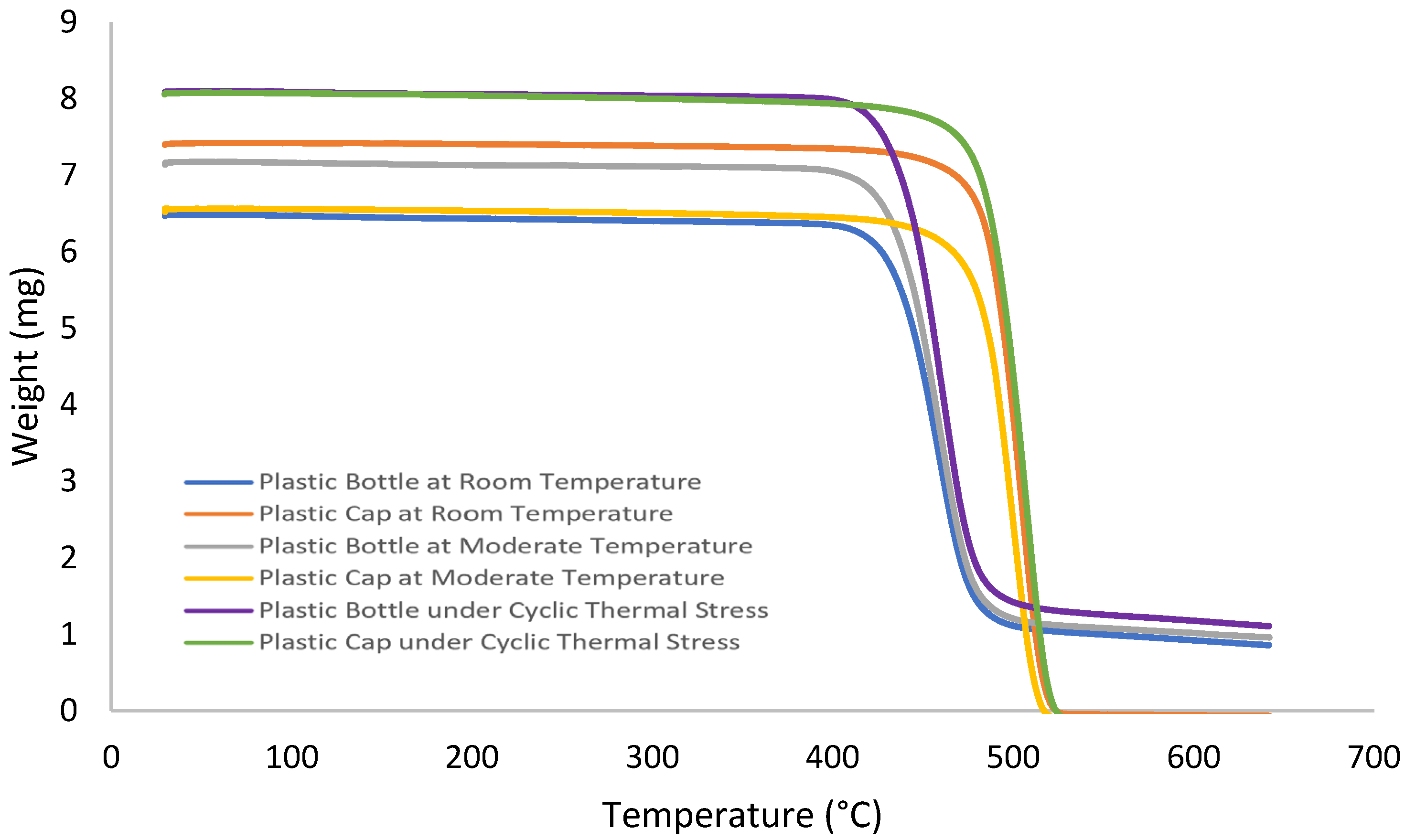
3.2. Thermal Analysis Result
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babaei, M.; Jalilian, M.; Shahbaz, K. Chemical recycling of Polyethylene terephthalate: A mini-review. J. Environ. Chem. Eng. 2024, 12, 112507. [Google Scholar] [CrossRef]
- Cao, F.; Wang, L.; Zheng, R.; Guo, L.; Chen, Y.; Qian, X. Research and progress of chemical depolymerization of waste PET and high-value application of its depolymerization products. RSC Adv. 2022, 12, 31564–31576. [Google Scholar] [CrossRef]
- Soong, Y.-H.V.; Sobkowicz, M.J.; Xie, D. Recent Advances in Biological Recycling of Polyethylene Terephthalate (PET) Plastic Wastes. Bioengineering 2022, 9, 98. [Google Scholar] [CrossRef]
- Bohre, A.; Tripathi, K.; Saha, B.; Likozar, B.; Pant, K.K.; Jadhao, P.R. Chemical Recycling Processes of Waste Polyethylene Terephthalate Using Solid Catalysts. ChemSusChem 2023, 16, e202300142. [Google Scholar] [CrossRef] [PubMed]
- Franz, R.; Welle, F. Contamination Levels in Recollected PET Bottles from Non-Food Applications and their Impact on the Safety of Recycled PET for Food Contact. Molecules 2020, 25, 4998. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.D.; Lopes, J.A.; Corredig, M. Chemical testing of mechanically recycled polyethylene terephthalate for food packaging in the European Union. Resour. Conserv. Recycl. 2021, 179, 106096. [Google Scholar] [CrossRef]
- Suhaimi, N.A.S.; Muhamad, F.; Abd Razak, N.A.; Zeimaran, E. Recycling of polyethylene terephthalate wastes: A review of technologies, routes, and applications. Polym. Eng. Sci. 2022, 62, 2355–2375. [Google Scholar] [CrossRef]
- Law, K.L.; Sobkowicz, M.J.; Shaver, M.P.; Hahn, M.E. Untangling the chemical complexity of plastics to improve life cycle outcomes. Nature Reviews. Materials 2024, 9, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Sokołowski, A.; Kończak, M.; Oleszczuk, P.; Gao, Y.; Czech, B. Environmental and Food Contamination by Phthalic Acid Esters (PAEs): Overview. Water Air Soil Pollut. 2024, 235, 313. [Google Scholar] [CrossRef]
- Kaewlaoyoong, A.; Liao, C.S.; Chen, J.-R.; Vu, C.T.; Lin, C. Occurrence of phthalate esters around the major plastic industrial area in southern Taiwan. Environ. Earth Sci. 2018, 77, 475. [Google Scholar] [CrossRef]
- Vincoff, S.; Schleupner, B.; Santos, J.; Morrison, M.; Zhang, N.; Dunphy-Daly, M.M.; Eward, W.C.; Armstrong, A.J.; Diana, Z.; Diana, Z.; et al. The Known and Unknown: Investigating the Carcinogenic Potential of Plastic Additives. Environ. Sci. Technol. 2024, 58, 10445–10457. [Google Scholar] [CrossRef]
- Baneshi, M.; Sabu Abraham, B.; Prosser, A.; Mkandawire, M.; Britten, A.J.; Tonney-Gagne, J.; Halilu, F.; Pilavangan, K.; Kanchanadevi Marimuthu, N.; Kaliaperumal, R. Unpacking Phthalates from Obscurity in the Environment. Molecules 2023, 29, 106. [Google Scholar] [CrossRef]
- Ahn, C.; Jeung, E.-B. Endocrine-Disrupting Chemicals and Disease Endpoints. Int. J. Mol. Sci. 2023, 24, 5342. [Google Scholar] [CrossRef] [PubMed]
- Onuzulu, C.D.; Rotimi, S.O.; Rotimi, O.A. Epigenetic modifications associated with in utero exposure to endocrine disrupting chemicals BPA, DDT and Pb. Rev. Environ. Health 2019, 34, 309–325. [Google Scholar] [CrossRef]
- Costa, E.M.F.; Spritzer, P.M.; Hohl, A.; Bachega, T.A.S.S. Effects of endocrine disruptors in the development of the female reproductive tract. Arq. Bras. De Endocrinol. Metabol. 2014, 58, 153–161. [Google Scholar] [CrossRef]
- Guarnotta, V.; Frasca, F.; Giordano, C.; Aversa, A.; Amodei, R. Impact of Chemical Endocrine Disruptors and Hormone Modulators on the Endocrine System. Int. J. Mol. Sci. 2022, 23, 5710. [Google Scholar] [CrossRef]
- Silva, A.B.P.; Carreiró, F.; Sanches-Silva, A.; Ramos, F. The role of endocrine disruptors in female infertility. Mol. Biol. Rep. 2023, 50, 7069–7088. [Google Scholar] [CrossRef]
- Encarnação, T.; Burrows, H.D.; Campos, M.G.; Pais, A.A. Endocrine disrupting chemicals: Impact on human health, wildlife and the environment. Sci. Prog. 2019, 102, 3–42. [Google Scholar] [CrossRef] [PubMed]
- Celik, Y.; Shamsuyeva, M.; Endres, H.J. Thermal and Mechanical Properties of the Recycled and Virgin PET-Part I. Polymers 2022, 14, 1326. [Google Scholar] [CrossRef] [PubMed]
- Viora, L.; Combeaud, C.; Pucci, M.F.; Perrin, D.; Bouvard, J.-L.; Combeau, M.; Liotier, P.-J. A Comparative Study on Crystallisation for Virgin and Recycled Polyethylene Terephthalate (PET): Multiscale Effects on Physico-Mechanical Properties. Polymers 2023, 15, 4613. [Google Scholar] [CrossRef] [PubMed]
- Sustaita-Rodríguez, J.M.; Gimenez, A.J.; Medellín-Rodríguez, F.J.; Olvera-Mendez, D.C.; Luna-Barcenas, G. Thermal Stability and Early Degradation Mechanisms of High-Density Polyethylene, Polyamide 6 (Nylon 6), and Polyethylene Terephthalate. Polym. Eng. Sci. 2019, 59, 2016–2023. [Google Scholar] [CrossRef]
- Alemadi, A.; Alani, M.A.; Akkbik, M.; Al-Qahtani, N. Unveiling Bottled Water Perils: Investigating Phthalate Ester Acid Leaching from Bottled Water in Qatar’s Scorching Climes. Mater. Proc. 2024, 18, 7. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ani, M.; Al-Qahtani, N. Melting Boundaries: How Heat Transforms Recycled Bottles into Chemical Time Bombs. Mater. Proc. 2025, 22, 8. https://doi.org/10.3390/materproc2025022008
Al-Ani M, Al-Qahtani N. Melting Boundaries: How Heat Transforms Recycled Bottles into Chemical Time Bombs. Materials Proceedings. 2025; 22(1):8. https://doi.org/10.3390/materproc2025022008
Chicago/Turabian StyleAl-Ani, Marwa, and Noora Al-Qahtani. 2025. "Melting Boundaries: How Heat Transforms Recycled Bottles into Chemical Time Bombs" Materials Proceedings 22, no. 1: 8. https://doi.org/10.3390/materproc2025022008
APA StyleAl-Ani, M., & Al-Qahtani, N. (2025). Melting Boundaries: How Heat Transforms Recycled Bottles into Chemical Time Bombs. Materials Proceedings, 22(1), 8. https://doi.org/10.3390/materproc2025022008




