Hybrid Oxidation of Titanium Substrates for Biomedical Applications †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- Diffusion oxidation in a fluidized bed (FB) leads to the formation of a highly defected Tiα(O) diffusion zone with good strength properties and nano-porous TiO2. Such a system plays a role as a foundation for the subsequent deposition of thin TiO2 layers by PVD magnetron sputtering.
- The hybrid oxidation treatment applies two types of surface activation, I: mechanical, as an impact of an aeromechanical factor in FB; II: sputtering, with simultaneous oxidation by PVD. Activation increases the number of active centers, and enhances oxygen mass transport to finally form homogenous thin TiO2 layers. The layers are characterized by a high level of homogeneity and resistance to cracking and delayering.
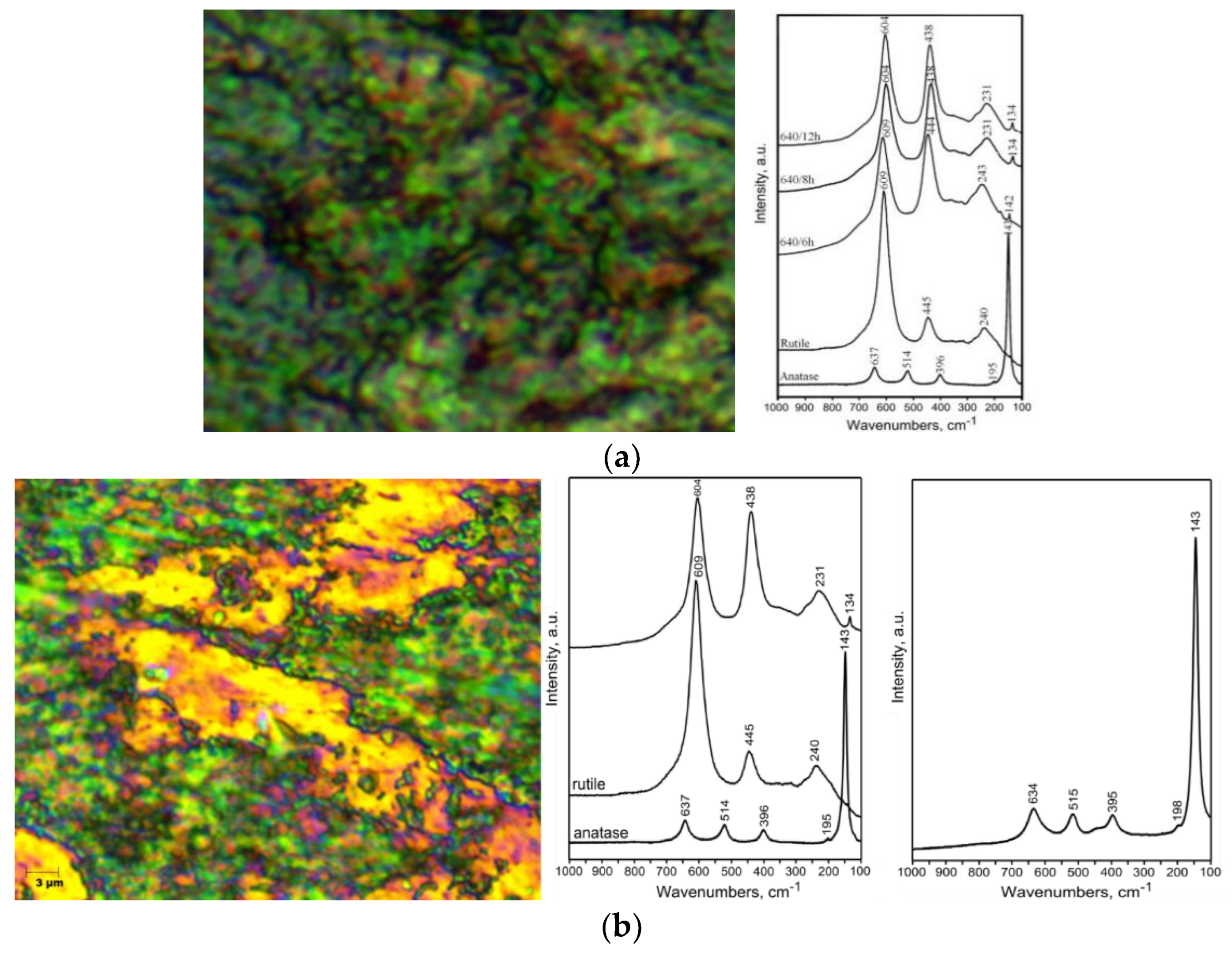
- In hybrid oxidation, the interface between nano-porous FB TiO2 and PVD TiO2 has a favorable state of stress and further influences the formation of a bioactive rutile and anatase mixture, which improves the rate of osseointegration.
- The presented hybrid oxidation is a promising surface treatment for biomedical applications, indicating the directions of forming bioactive layers on titanium substrates. The solution corresponds with the new trends in biomaterials and surface engineering to combine different processing techniques in order to improve implants and medical devices.
5. Patents
Funding
Conflicts of Interest
References
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.M.; Kawashita, M.; Nakamura, T. Bioactive metals: preparation and properties. J. Mater. Sci. Mater. Med. 2004, 15, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, N.; Pax, R.A.; Mac, A.; Gray, E. Review of functional titanium oxides. I: TiO2 and its modifications. Prog. Solid State Chem. 2016, 44, 86–105. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, X.; Shen, R.; Rogachev, A.V. Preparation and characterization of TiO2 thin film by thermal oxidation of sputtered Ti film. Mater. Sci. Semicond. Process. 2013, 16, 513–519. [Google Scholar] [CrossRef]
- Radmanesh, M.; Kiani, A. Bioactivity enhancement of titanium induced by Nd:Yag laser pulses. J. Appl. Biomater. Funct. Mater. 2016, 14, 70–77. [Google Scholar]
- Wu, B.; Yu, Y.; Wu, J.; Shchelkanov, I.; Ruzic, D.N.; Huang, N.; Len, Y.X. Tailoring of titanium thin film properties in high power pulsed magnetron sputtering. Vacuum 2018, 150, 144–154. [Google Scholar] [CrossRef]
- Heinrichs, J.; Jarmar, T.; Wiklund, U.; Engqvist, H. Physical Vapour Deposition and Bioactivity of Crystalline Titanium Dioxide Thin Films. Trends Biomater. Artif. Organs 2008, 22, 104–110. [Google Scholar]
- Shannon, R.D.; Pask, J.A. Kinetics of the anatase-rutile transformation. J. Am. Ceram. Soc. 1965, 48, 391–398. [Google Scholar] [CrossRef]
- Aniołek, K. The influence of thermal oxidation parameters on the growth of oxide layers on titanium. Vacuum 2017, 144, 94–100. [Google Scholar] [CrossRef]
- Satoh, N.; Nakashima, T.; Yamamoto, K. Metastability of anatase: size dependent and irreversible anatase-rutile phase transition in atomic-level precise titania. Sci. Rep. 2013, 3, 1959. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.S.; Sahoo, S.; Pradhan, S.K. Influence of annealing temperature on the structural, mechanical and wetting property of TiO2 films deposited by RF magnetron sputtering. Thin Solid Films 2010, 518, 6904–6908. [Google Scholar] [CrossRef]
- Ochsenbein, A.; Chai, F.; Winter, S.; Traisnel, M.; Breme, J.; Hildebrand, H.F. Osteoblast responses to different oxide coatings produced by the sol-gel process on titanium substrates. Acta Biomater. 2008, 4, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Barfeie, A.; Wilson, J.; Rees, J. Implant surface characteristics and their effect on osseointegration. Br. Dent. J. 2015, 218, E9. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 38883–38903. [Google Scholar] [CrossRef]
- Forsgren, J.; Svahn, F.; Jarmar, T.; Engqvist, H. Formation and adhesion of biomimetic hydroxyapatite deposited on titanium substrates. Acta Biomater. 2007, 3, 980–984. [Google Scholar] [CrossRef]
- Rosales-Leal, J.I.; Rodríguez-Valverde, M.A.; Mazzaglia, G.; Ramon-Torregrosa, P.J.; Diaz-Rodriguez, L.; Garcia-Martinez, O.; Vallecillo-Capilla, M.; Ruiz, C.; Cabrerizo-Vilchez, M.A. Effect of roughness, wettability and morphology of engineered titanium surfaces on osteoblast-like cell adhesion. Colloids Surf. A Physicochem. Eng. Asp. 2010, 365, 222–229. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Nath, S.; Sugawara, Y.; Divakarla, K.; Das, T.; Manos, J.; Chrzanowski, W.; Matsushita, T.; Kokubo, T. Two-in-one biointerfaces—Antimicrobial and bioactive nanoporous gallium titanate layers for titanium implants. Nanomaterials 2017, 7, 229. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, X.; Yue, P.L.; Lu, G.Q.; Greenfield, P.F. Synthesis of anatase TiO2 supported on porous solids by chemical vapor deposition. Catal. Today 2001, 68, 173–182. [Google Scholar] [CrossRef]
- Sabetrasekh, R.; Tiainen, H.; Lyngstadaas, S.P.; Reseland, J.; Haugen, H. A novel ultra-porous titanium dioxide ceramic with excellent biocompatibility. J. Biomater. Appl. 2011, 25, 559–580. [Google Scholar] [CrossRef]
- Sengottuvelan, A.; Balasubramanian, P.; Will, J.; Boccaccini, A.R. Bioactivation of titanium dioxide scaffolds by ALP-functionalization. Bioact. Mater. 2017, 2, 108–115. [Google Scholar] [CrossRef]
- Li, D.; Ferguson, S.J.; Beutler, T.; Cochran, D.L.; Siting, C.; Hirt, H.P.; Buser, D.J. Biomechanical comparison of the sandblasted and acid-etched and the machined and acid-etched titanium surface for dental implants. J. Biomed. Mater. Res. 2002, 60, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Lubas, M.; Sitarz, M.; Jasinski, J.J.; Jelen, P.; Klita, L.; Podsiad, P.; Jasinski, J. Fabrication and characterization of oxygen–Diffused titanium using spectroscopy method. Spectrochim. Acta A 2014, 133, 883–886. [Google Scholar] [CrossRef]
- Sarvadii, S.Y.; Gatin, A.K.; Kharitonov, V.A.; Dokhlikova, N.V.; Ozerin, S.A.; Grishin, M.V.; Shub, B.R. Oxidation of Thin Titanium Films: Determination of the Chemical Composition of the Oxide and the Oxygen Diffusion Factor. Crystals 2020, 10, 117. [Google Scholar] [CrossRef]
- Toptan, F.; Alves, A.C.; Pinto, A.M.P.; Ponthiaux, P. Tribocorrosion behavior of bio-functionalized highly porous titanium. J. Mech. Behav. Biomed. 2017, 69, 144–152. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhou, W.; Zhou, X.; Zhong, X.; Zhang, X.; Wan, P.; Zhu, B.; Chen, W. The anatase phase of nanotopography titania plays an important role on osteoblast cell morphology and proliferation. J. Mater. Sci. Mater. Med. 2008, 19, 3465–3472. [Google Scholar] [CrossRef]
- Jasinski, J.J.; Lubas, M.; Kurpaska, L.; Napadlek, W.; Sitarz, M. Functionalization of Ti99.2 substrates surface by hybrid treatment investigated with spectroscopic methods. J. Mol. Struct. 2018, 1164, 412–419. [Google Scholar] [CrossRef]
- Pang, M.; Bahr, D. Thin-film fracture during nanoindentation of a titanium oxide film–titanium system. J. Mater. Res. 2001, 16, 2634–2643. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity. Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Oyane, A.; Onuma, K.; Ito, A.; Kim, H.M.; Kokubo, T.; Nakamura, T. Formation and growth of clusters in conventional and new kinds of simulated body fluids. J. Biomed. Mater. Res. 2003, 64, 339–348. [Google Scholar] [CrossRef]


| Material | Chemical Composition | |||||
|---|---|---|---|---|---|---|
| KOBE Steel LTD Titanium Grade 2 (ASTM 8348) | O | N | C | H | Fe | Ti |
| 0.20 | 0.03 | 0.10 | 0.015 | 0.30 | rest | |
| Substrate Type | Hardness, H (GPa) | Reduced Young’s Modulus, ER (GPa) | Calculated Young’s Modulus, E (GPa) | Maximum Depth (nm) | Plastic Depth (nm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Titanium Grade 2 (ASTM 8348) | Value | SD | Value | SD | Value | SD | Value | SD | Value | SD |
| 9.33 | 4.14 | 160.00 | 60.30 | 148.34 | 55.91 | 204.08 | 57.41 | 167.17 | 54.36 | |
| Titanium after hybrid oxidation FB + PVD | Value | SD | Value | SD | Value | SD | Value | SD | Value | SD |
| 15.21 | 6.04 | 281.83 | 87.79 | 261.28 | 81.39 | 144.90 | 28.87 | 119.20 | 27.37 | |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasinski, J.J. Hybrid Oxidation of Titanium Substrates for Biomedical Applications. Mater. Proc. 2020, 2, 8. https://doi.org/10.3390/CIWC2020-06845
Jasinski JJ. Hybrid Oxidation of Titanium Substrates for Biomedical Applications. Materials Proceedings. 2020; 2(1):8. https://doi.org/10.3390/CIWC2020-06845
Chicago/Turabian StyleJasinski, Jaroslaw Jan. 2020. "Hybrid Oxidation of Titanium Substrates for Biomedical Applications" Materials Proceedings 2, no. 1: 8. https://doi.org/10.3390/CIWC2020-06845
APA StyleJasinski, J. J. (2020). Hybrid Oxidation of Titanium Substrates for Biomedical Applications. Materials Proceedings, 2(1), 8. https://doi.org/10.3390/CIWC2020-06845





