1. Introduction
In recent years, the electrospinning (ES) technique has received a lot of attention in the scientific field for manufacturing morphologically useful substrates for application in tissue engineering (TE). Natural fibers in ECM have nanometric or submicrometric dimension and with ES it is possible to produce fibrous matrices with nanometric fibers made of several different polymers [
1,
2,
3]. Among the suitable polymers, those of natural origin are of particular interest for biomimetic approaches in TE since they offer the advantage of reduced risk for tissue toxicity or inflammatory host immune responses. In particular, silk fibroin (SF), a protein produced by lepidoptera or spiders, is characterized by a highly repetitive primary sequence of amino-acids that provides a high degree of crystallinity and central mechanical properties [
4,
5]. These properties, joined to the excellent biocompatibility and environmental stability of SF, provide a great opportunity in biomedical applications. Among the various techniques useful to process silk fibroin, ES offers an interesting opportunity for producing 2D and 3D matrices with great potential for tissue regeneration and repair [
6,
7,
8]. Electrospun SF was applied to the production of scaffolds for a variety of biological applications such as bone, nerve and skin tissue [
9]. In some cases, however, ES scaffold produced with a single polymer may be inadequate in providing the appropriate mechanical and biological properties for regeneration of specific tissues, particularly in applications in contact with blood, e.g., in blood vessel substitutes [
10,
11].
Hybridization [
12,
13,
14] or coating with biomacromolecules [
15] can improve the performance of electrospun 3D scaffolds and 2D matrices. The hybridization of electrospun SF and type I collagen was proposed to combine the advantages intrinsic in each material and minimize their drawbacks [
12].
However, collagen is potentially immunogenic because of its animal origin, and it presents high costs [
16]. To overcome these limitations, gelatin can be used as an alternative [
17]. Gelatin is an inexpensive natural polymer derived from a partial hydrolysis of collagen; it is non-immunogenic, biodegradable, easy to process and biocompatible. Such a protein also has the natural cell binding motifs like arginine-glycine-aspartic acid (RGD) that is favorable for cell activities. However, gelatin is rarely used alone owing to its high weakness, and thus needs to be modified with several methods including crosslinking, grafting and blending.
Blends of silk fibroin and gelatin polymers have been electrospun to produce scaffolds for vascular tissue engineering or drug delivery systems.
Electrospun SF/gelatin nanofiber mats, as blends, have already been developed and evaluated [
18,
19]; however, this approach has some intrinsic limitations, as gelatin needs to be stabilized by a crosslinking reaction that may alter fiber morphology alteration or other stability and/or toxicity problems.
In this work, a gelatin coating was applied to electrospun silk fibroin (ESF) mats and tubes intended for the regeneration of cardiovascular tissues. The crosslinking reaction used is based on a Michael-type addition in water that promotes the formation of covalent bonds between gelatin amino groups and β-carbons of N-N’-methylene bis-acrylamide (MBA). MBA crosslinking was easily obtained in situ by loading or dipping the ESF samples with the crosslinking solution, by use of static or dynamic homemade systems.
Morphology, water uptake, stability and tensile properties of 2D and 3D (tubular) gelatin-coated samples were evaluated and cytocompatibility was successfully assessed with L929 and primary Human Umbilical cord Vein Endothelial Cells.
2. Materials and Methods
2.1. Preparation of 2D and 3D Gelatin Coated ESF Samples
Electrospun silk fibroin (ESF) plane and tubular structures were fabricated by electrospinning, with a homemade apparatus, using a 7.5%
w/v SF solution in formic acid, a plane (plate of 10 × 10 cm) or a rotating mandrel (Ø = 6 mm, l = 10 cm) stainless steel collector, with previously described optimized parameters [
7,
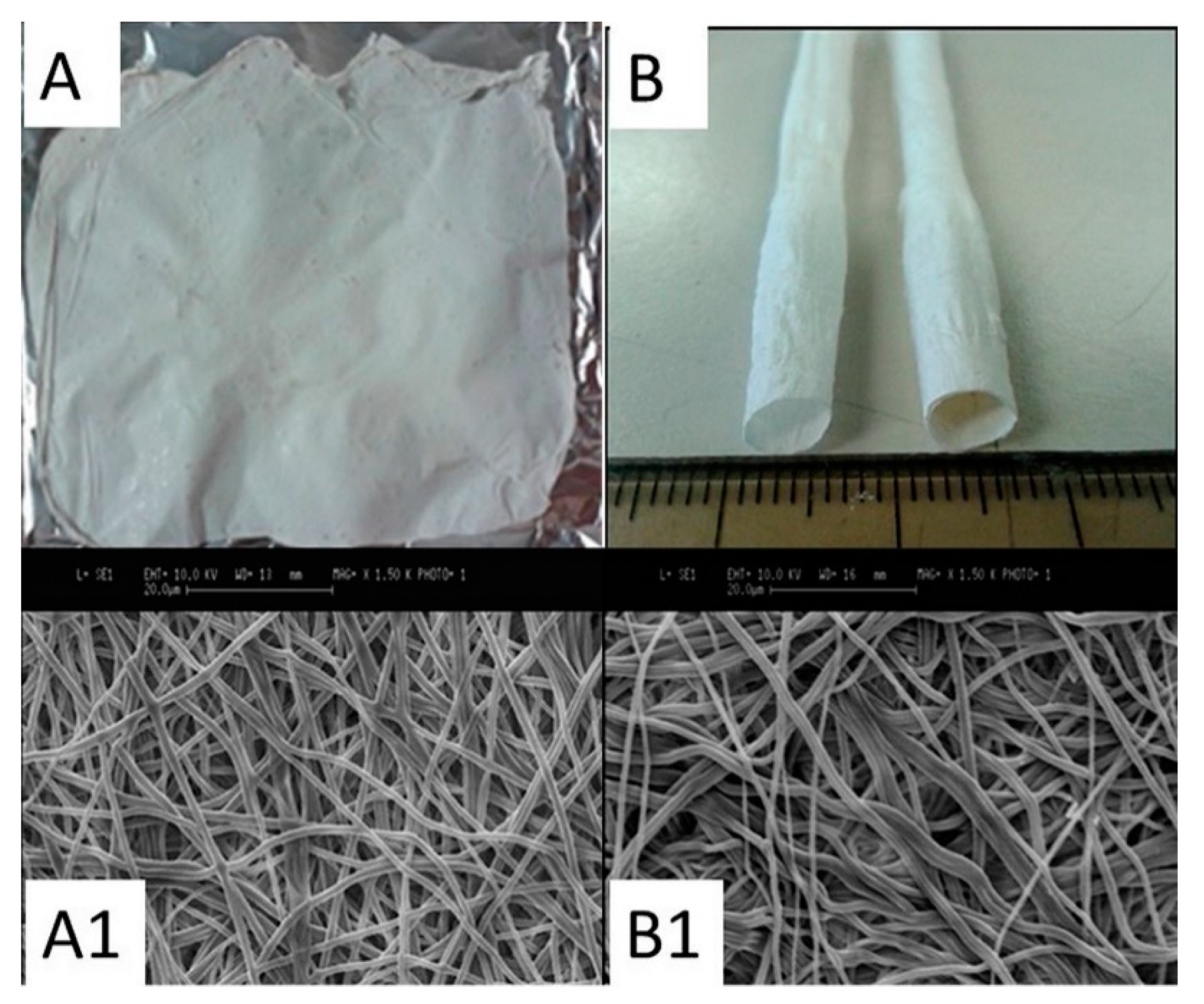
8]. ESF samples were treated with methanol (>99.9%) to induce silk fibroin crystallization. Images of the ESF structures are presented in
Figure 1.
The electrospun SF samples were coated with type A gelatin crosslinked in situ by a Michael-type addition reaction that creates covalent bonds between gelatin amino groups and β-carbons of N-N′-methylene bis-acrylamide (MBA). The gel is prepared by dissolving 1 g of gelatin (from porcine skin, G1890 Sigma-Aldrich) in 6 mL of distilled water at 50 °C, adding 20 μL of triethylamine to increase the pH to 10 and then MBA (23.3 mg) as crosslinking agent [
20]. Completion of the crosslinking reaction requires 24 h at 50 °C.
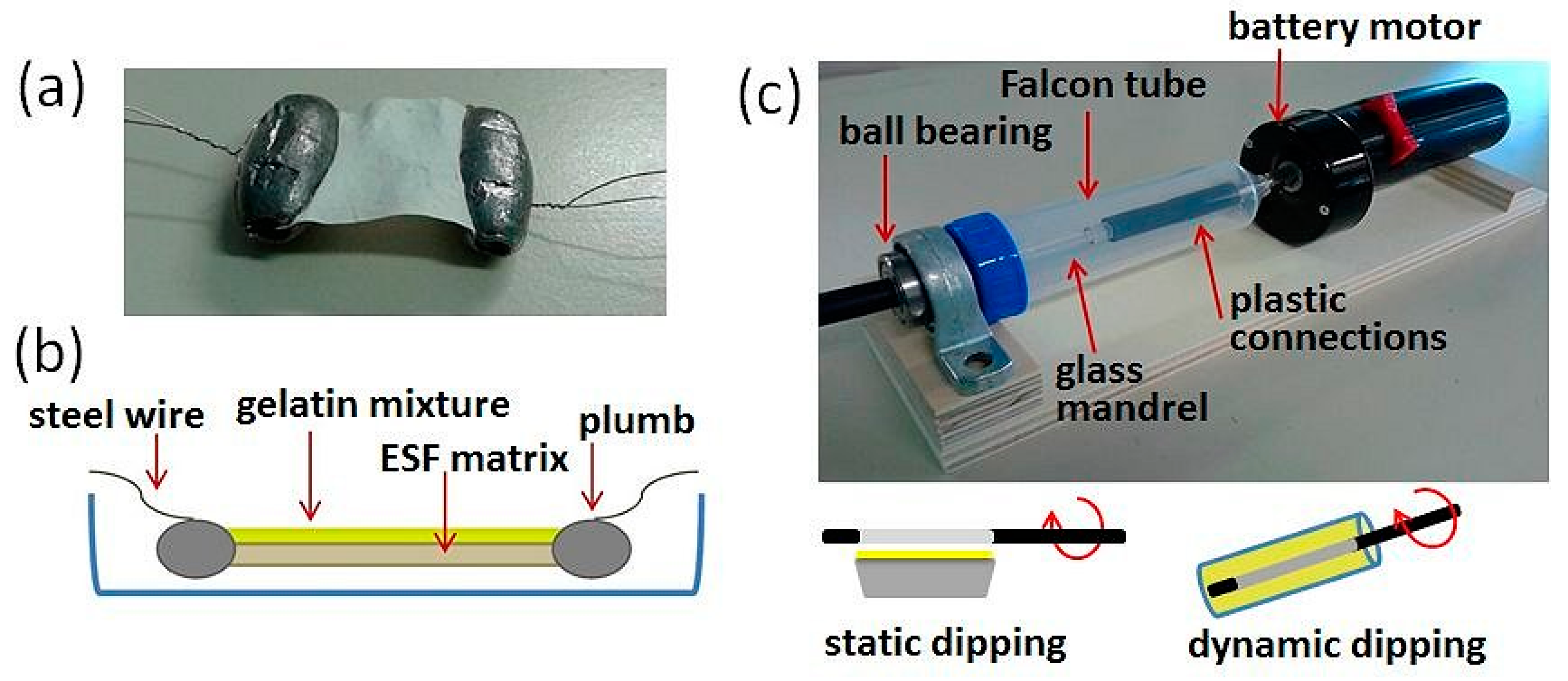
To obtain a homogeneous coating on the ESF substrates, different methods were tested. The 2D sample mat was kept taut and suspended on a glass container by fixing the ends with plumbs in turn anchored to the container walls with a stainless steel wire (
Figure 2a,b). To proceed with the coating (
Figure 2b), the crosslinking solution was deposited on the ESF mat using a plastic syringe, then placing the system in an oven for 24 h at 50 °C.
The system specifically set up for 3D ESF substrates (
Figure 2c) is composed of a battery motor resistant to high temperatures, which, through a steel shaft and a rubber joint, transmits the rotation to a glass bar on which an electrospun fibroin tube had been previously mounted (
Figure 2c). The rotation speed of the system generated by the motor is 5 rpm. To ensure a correct transmission of motion from the rotating shaft to the glass bar, couplings between polymeric materials were selected to avoid corrosion and wear problems. The system composed of glass bar, plastic fittings and rotating metal shaft is inserted into a 50 mL Falcon tube drilled at the ends to allow the connection to the rotating shaft on one side and on the other the insertion in a support for ball bearings used to keep the system in axis without interfering with the rotation (
Figure 2c).
Different approaches were investigated for the 3D fibroin structure coating:
- I.
“static dipping”: deposition by contact between the outer surface of the fibroin tube and the solution of gelatin placed in a preheated aluminum container, provided with housings necessary for inserting the ESF tube mounted on the spindle.
- II.
“dynamic dipping”: dipping of the ESF tube kept in motion by the automatic system into the gelatin solution. The gelatin solution is placed in a preheated cylindrical plastic container (
Figure 2c), and the ESF tube, preheated and mounted on the system, is coated by dipping inside this container, while it is kept rotating.
After the gelatin impregnation, to obtain a homogeneous coating in a confined environment, each system is transferred inside the Falcon tube that is sealed at the extremities, and housed in the device, kept in constant rotation and placed in an oven at 50 °C for 24 h to allow for the complete gelatin crosslinking.
Finally, all gelatin-coated ESF samples were purified by consecutive washing steps with pure ethanol, hydrochloric acid (HCl) 0.1 M and distilled water to eliminate possible unreacted MBA and low molecular weight substances. The coated samples were then dehydrated by dipping in ethanol solutions and stored at room temperature under reduced pressure until analysis.
2.2. Characterization of Coated Samples
The wall thickness of uncoated and coated ESF samples (n = 7) after swelling in water was measured with a micrometer (Somet).
Morphology was evaluated by scanning electron microscopy (SEM, StereoScan 360, Cambridge Instruments, Cambridge, UK). The average fiber diameter was calculated from SEM images acquired on five different samples; for each sample, 20 measures were acquired with ImageJ v.1.42q software (NIH, Bethesda, MD, USA). For SEM analysis, gelatin-coated samples were dehydrated in graded ethanol solutions, sputter-coated with gold (Edwards Sputter Coater 5150B, UK) and observed with a 10 keV accelerating voltage.
Swelling and weight loss in demineralized water at 37 °C were evaluated on five specimens per sample type. Wet sample weight at different time points (up to 25 days) was determined by blotting the sample surface with filter paper to remove excess water and then immediately weighing. The weight variation of the samples was calculated following Equation (1):
where
Wi is the sample weight at time point
i and
Wo is the initial dry weight of the sample.
Weight variation of the samples for each test time is represented by the average value and standard deviation of data obtained from five specimens of the same type.
Circumferential tensile mechanical tests were carried out on cylindrical specimens (5 mm length segments) of coated and uncoated ESF tubes. The samples (n = 5 for each test and material) previously brought to the swelling plateau in distilled water at 37 °C were housed between two L-shaped stainless-steel grips.
The tests were performed by use of a dynamic-mechanical analyzer (DMA, Q800, TA Instruments, New Castle, DE, USA), with a preload of 0.005 N and a force ramp of 0.5 N/min up to sample break. From the obtained stress/strain curves, the evaluated parameters were elastic modulus (E), ultimate tensile stress (σb) and strain at break (εb).
2.3. Cytocompatibility Tests
For in vitro interaction tests with L929 cells (murine fibroblasts, ECACC n° 85011425) circular specimens of gelatin-coated ESF samples punched from the tubular scaffolds (Ø = 6 mm, uncoated ones as control) were disinfected with a 70% ethanol solution under a laminar flow hood for 1 h and left to dry in sterile conditions for 24 h.
Indirect cytotoxicity tests were carried out to verify the possible release of toxic products and consisted in culturing the cells in the presence of extracts (eluates) of the samples. The eluates were obtained by immersing the samples (n = 3, per time point) in complete Dulbecco’s Modified Eagle Medium (DMEM, Sigma Aldrich, Merck Life Science S.r.l., Milano, Italy) for 1, 3 and 7 days. L929 cells (density = 1 × 104 cells/well) were seeded in the 96-multiwell culture plastic (TCPS) and cultured for 24 h to allow for cell adhesion on the well bottom. After that, the medium was replaced with the obtained DMEM eluates, using DMEM only as control. After 24 h of culture, cell viability was investigated with the MTT colorimetric assay, based on the use of the MTT tetrazolium salt [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide)] that is reduced to a purple insoluble formazan product in living cells by mitochondrial succinate dehydrogenase. The absorbance was evaluated at 570 nm with a spectrophotometer (TECAN, Genios Plus, Männedorf, Switzerland).
Direct contact tests were performed with Human umbilical cord vein endothelial cells (HUVEC) on crosslinked gelatin samples and gelatin-coated ESF mats (uncoated ones as control). Primary cultures of HUVEC were obtained through enzymatic digestion of the human umbilical cord vein provided by the Cittadella Hospital (Verona) and processed within 24 h of delivery.
Specimens having a diameter of 6 mm were obtained with a manual die (n = 3), washed under a laminar flow hood in a 50% ethanol solution for 2 h, in pure ethanol for a further 2 h and then allowed to dry overnight in sterile conditions. Subsequently, the specimens were transferred to 96-well TCPS plates.
Then, 5 × 103 HUVECs per well were seeded on each sample and allowed to adhere and proliferate for up to 7 days under standard tissue culture conditions.
In the case of gelatin gels, at each time point (1, 3 and 7 days), non-adherent cells were removed by rinsing with PBS. The adherent cells were fixed with 4% formalin overnight and stained with 0.5% (w/v in 20% ethanol) toluidine blue. Cell adhesion on the matrices was qualitatively evaluated by optical microscopy.
Cell proliferation on silk fibroin-based matrices was quantitatively evaluated from the total protein content obtained by lysis of the cells grown for 7 days on them; adherent cells were treated with 0.1% Triton X100 overnight at 4 °C to cause their lysis and the total protein content was determined using BCA Protein Assay. Absorbance was measured at 570 nm and results expressed in μg/mL, by use of a calibration curve obtained with bovine serum albumin.
2.4. Statistical Analysis
Data are presented as mean values ± standard deviation and statistically compared by two-tailed t-Test. Differences between groups were considered significant for p ≤ 0.05.
3. Results and Discussion
The morphology of the 2D matrices and electrospun tubular SF structures was analyzed by SEM observation. SEM images show the electrospun structure formed by randomly arranged fibers, typical of non-woven materials (
Figure 1(A1,B1)). No presence of defects (beads) due to the electrospinning process can be found. Furthermore, both mats and tubes exhibit a similar morphology of the internal surface, i.e., the surface in contact with the collector, and the external one.
Fiber dimensions, calculated using ImageJ software, are overall homogeneous with the same matrix (flat or tubular) and surface area considered (internal vs external), with values in the range 400–1200 nm following a bell-shaped distribution, and a mean value of less than 900 nm (
Table 1).
At SEM, it was possible to qualitatively assess the degree of penetration of the cross-linked gelatin gel inside the thickness of the electrospun 2D mat although the gelatin deposition took place only on one of the two surfaces. In the image shown in
Figure 3b, it is possible, in fact, to observe a homogeneous and compact layer of gelatin on both surfaces. In addition, (
Figure 3a) the nanofiber structure was barely distinguishable under the homogeneous coating of the crosslinked gelatin gel. The typical porosity of electrospun structures therefore allows the passage of gelatin through the entire thickness of the 2D matrix.
Consequently, also for the tubular matrix the coating procedure was applied from the external surface, thus obtaining the complete penetration of the crosslinked gel.
As shown in
Figure 3c,d, in both static and dynamic dipping, on the coated surfaces it is possible to note the presence of gelatin uniformly distributed among the fibers. In particular, after the “static dipping”, the nanofiber morphology of the electrospun structure was still evident (
Figure 3c), while the “dynamic dipping”, qualitatively, produces a coating with a greater thickness of gelatin (
Figure 3d). Moreover, the fibers are less evident than after the “static” method.
As a more efficient method, the “dynamic dipping” was therefore chosen for coating the ESF tubes with cross-linked gelatin.
From the measures performed with the micrometer, a significant difference (
p < 0.05) was observed between the wall thickness of the ESF tubes and that of the ESF mat, both in the anhydrous state (data not shown) and at the swelling plateau (
Table 1). This is attributable to the different surface of the collector (72 cm
2 for mats and 18.85 cm
2 for tubes) for the same volume of the fibroin solution used for electrospinning.
By comparing the thickness in wet conditions of the coated samples with the uncoated ones (
Table 1), it is possible to highlight a statistically significant difference (
p < 0.05), with an increase of thickness higher that 40%.
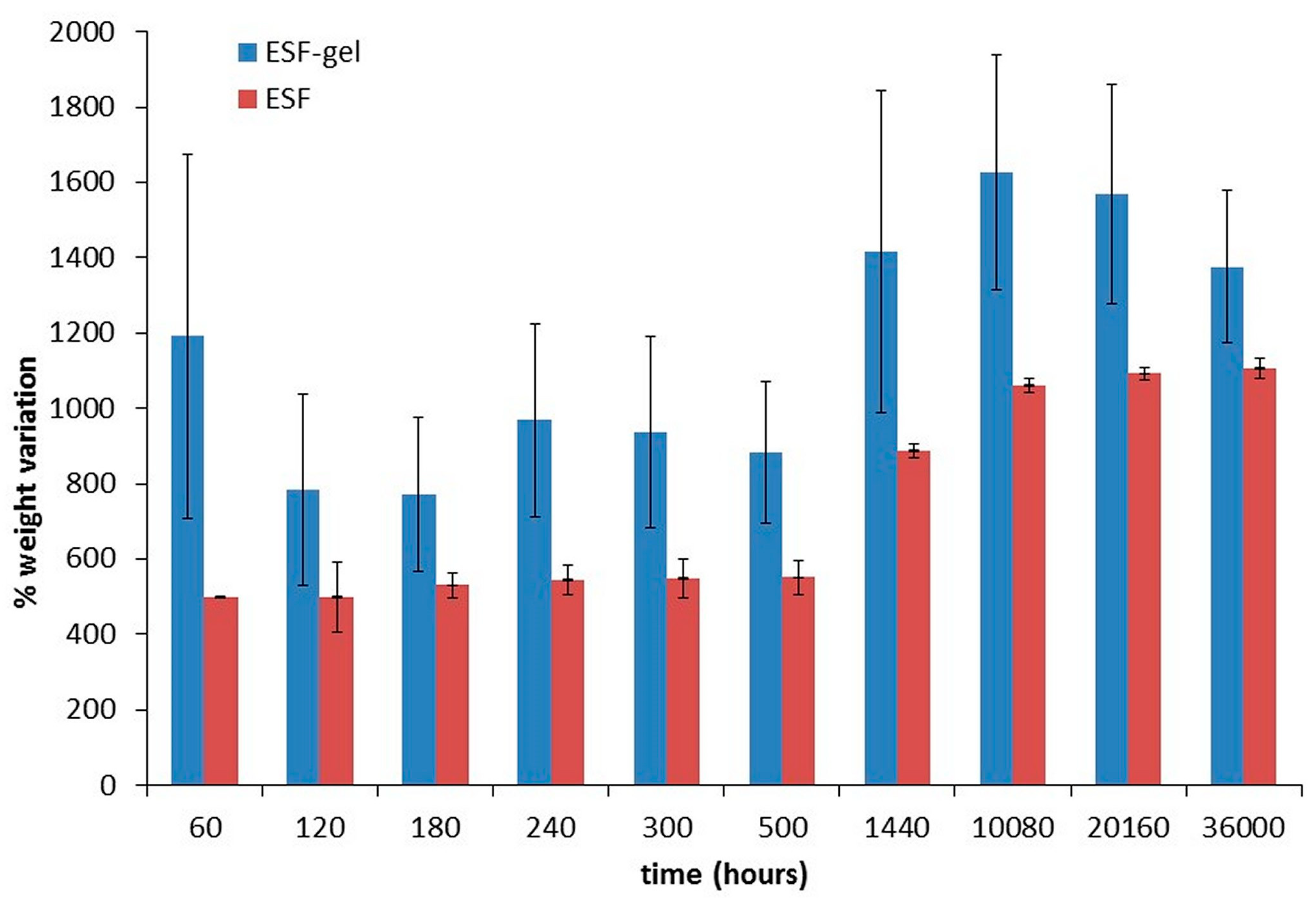
Weight variation tests, performed at 37 °C in water on 5 mm length tubular specimens indicated for ESF samples a rapid swelling within the first 10 min of incubation, reaching their maximum water uptake (≈500%) and maintaining this value of plateau until the 25th day (
Figure 4, red bars). The coated samples (ESFgel), instead, show higher swelling values (
p < 0.05), reaching an increase of 1000% after 60 min; subsequently, the weight variation still increases to 1600% up to 14 days and then starts decreasing (
Figure 4, blue bars), probably due to the beginning of the gelatin gel degradation.
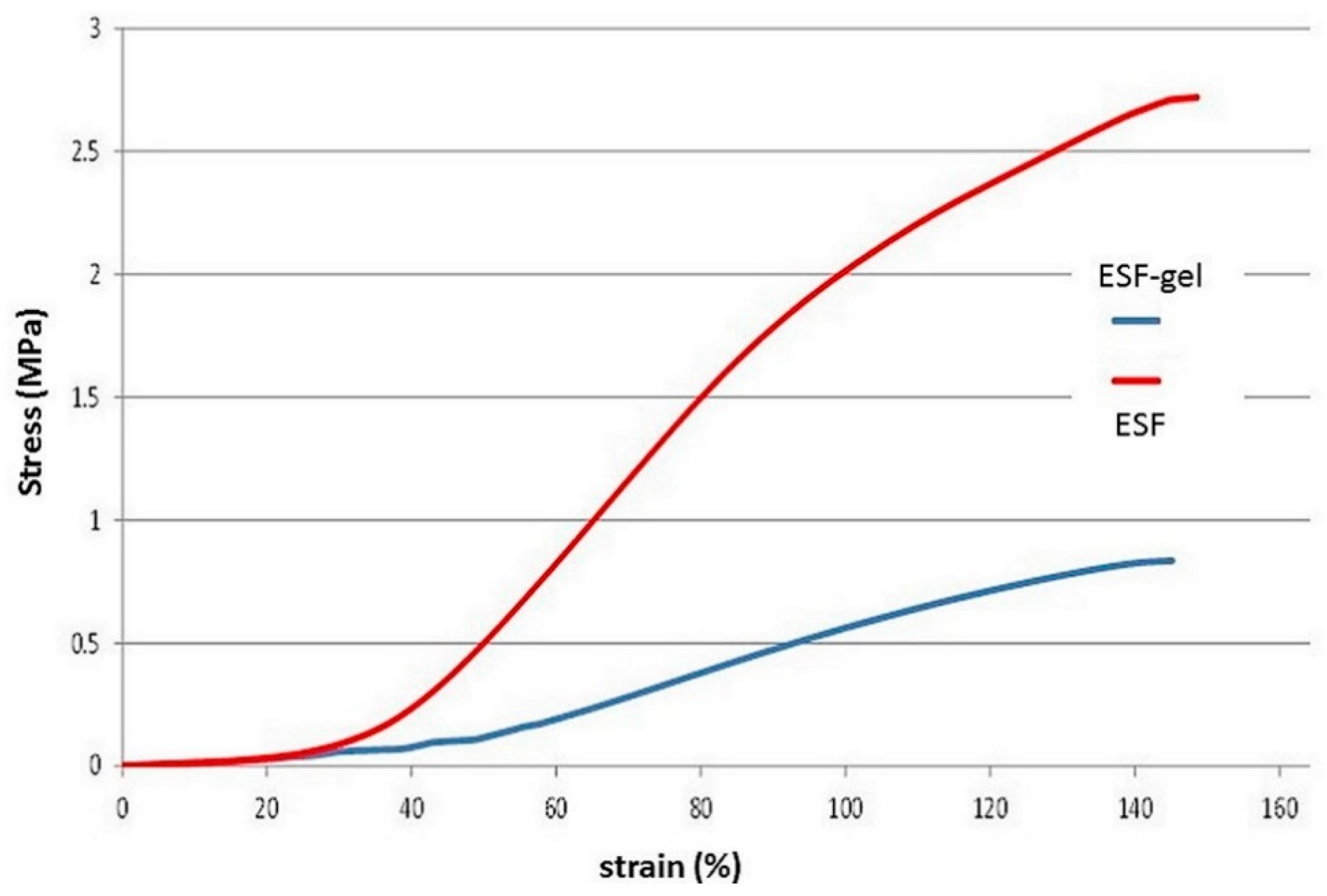
A comparison between circumferential tensile properties of gelatin-coated ESF tube versus non coated samples is provided by representative stress/strain curves shown in
Figure 5. The values of elastic modulus (E), stress (σ
b) and deformation (ε
b) at break are reported in
Table 1.
It is possible to notice how, with the same deformation, the corresponding stress values for ESF-gel are lower than those found for uncoaed ESF, indicating a lower stiffness. This observation is confirmed by the values of elastic modulus, significantly different by comparing the gelatin-coated samples with those of fibroin alone (p < 0.05).
The lower mechanical characteristics for ESF-gel are probably attributable to the gelatin coating, which balances the greater mechanical properties of fibroin alone.
In Vitro Interaction Tests with Cells
The MTT assay performed after 24 h of contact of the L929 cells with the culture medium in contact with the samples (
Figure 6) showed that, in the one-day eluates, the cell viability levels were significantly higher (
p < 0.05) for fibroin eluates (ESF) with respect to the control and a viability comparable to the control for ESF-gel eluates. For eluates after 3 days, the same trend was even more evident. At 7 days, however, the viability of the cells in contact with the ESF gel eluates increased considerably and became comparable (
p > 0.05) with that of the ESF samples. Both these values were higher than the control (
p < 0.05). Controls were represented by DMEM only, left in the incubator for the same periods of time (1, 3 and 7 days).
It is therefore possible to conclude that neither the electrospun fibroin nor the coating obtained by crosslinking gelatin with MBA release toxic substances that could decrease cell viability.
Direct cytocompatibility results with L929 cells were described in reference [
21], where the cells were seeded on samples prepared as for the indirect cytotoxicity tests and cultured for up to 7 days.
Alamar Blue assay indicated a good cytocompatibility for both uncoated and coated ESF samples with a linear increase of cell viability with culture time (1, 3 and 7 days), cell viability being comparable to that observed on the culture plastic (TCPS). At SEM, the cells were found spread and flattened already after the first day, and at day 7 their number appeared greater on gelatin-coated samples.
In this work, direct culture tests were performed with primary human endothelial cells (HUVEC). HUVEC were isolated from human umbilical cord vein, processed within 24 h of delivery, seeded and cultured up to 7 days on both crosslinked gelatin alone and ESF-coated samples.
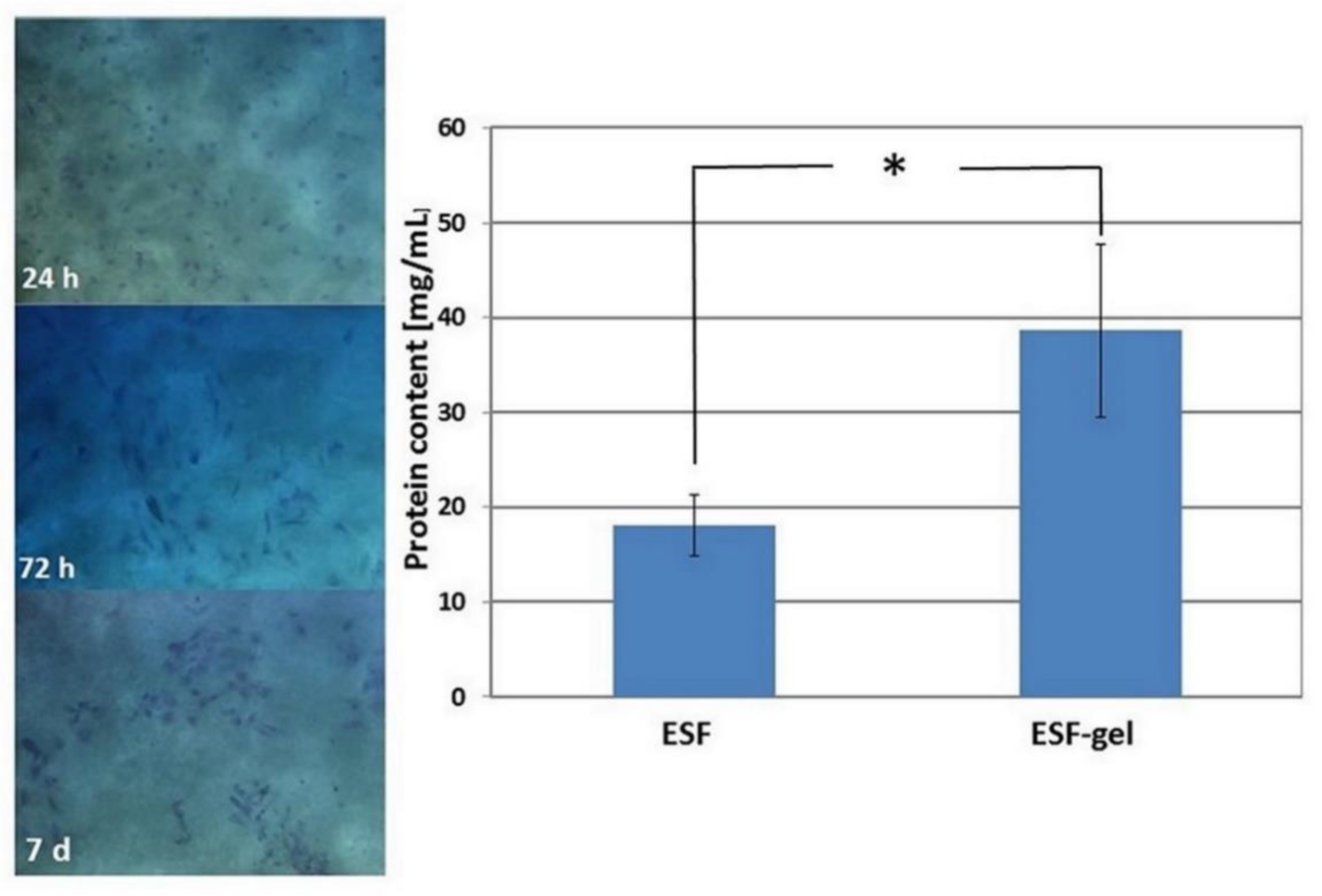
After the first day of culture on the matrix of gelatin, many viable cells both rounded and adherent were visible; over time, they tended to form organized colonies (representative OM images are provided in
Figure 7, left). Therefore, these results suggest that gelatin crosslinked with MBA represents a favorable environment for endothelial cells.
The evaluation of HUVEC growth on silk fibroin-based supports was carried out by quantitative determination of the total proteins in cell lysates. It can be observed (
Figure 7, right) that gelatin-coated ES fibroin displayed a higher total protein content than uncoated ES fibroin (
p < 0.05), therefore indicating a better cell growth on this type of matrix.
4. Conclusions
The novel 2D and 3D ESF-gel structures developed in this work demonstrated adequate morphological properties to support cell colonization, combined with good stability in an aqueous environment and acceptable mechanical properties, with an increase in flexibility compared to the structure of electrospun fibroin.
The crosslinking method developed overcomes gelatin limited stability at 37 °C, as the gel coating proved to be stable up to 14 days while maintaining good cell compatibility, which is therefore not significantly affected by the crosslinking reaction.
Interestingly, when the crosslinking mixture is applied to a substrate containing primary or even secondary amino groups, these groups can participate in the reaction, being incorporated into the gelatin coating, thus increasing the coating stability on the surface.
The per se remarkable biocompatibility of SF is therefore combined with a coating that acts as a sealant and is capable to promote cell adhesion and proliferation.