An Intelligent System for Predicting the Methanol Conversion Rate from the Direct Hydrogenation of CO2 under Uncertainty †
Abstract
1. Introduction
2. Process Description
3. Methodology
- First-Principles Model: A first-principles model for methanol synthesis was developed using Aspen Hysys (https://www.aspentech.com/en/products/engineering/aspen-hysys, accessed on 1 April 2024). Model accuracy and real-world relevance were ensured through the incorporation of literature data [3,12].
- Data Generation: Transitioning the Aspen Hysys model into dynamic mode, an interface between MATLAB and Aspen Hysys was established using actxserver. This dynamic mode introduced a ±5% uncertainty in critical process conditions (temperature, pressure, and mass flow rate), simulating real-world variability and resulting in a dataset of 370 samples. Table 1 presents some samples of generated data. Sample-1 represents the steady-state conditions of the Aspen Hysys model, while the rest were generated by inserting artificial uncertainty.
- 3.
- Soft Sensor Development: In the subsequent phase, a Matern GPR model served as a soft sensor and was developed using MATLAB 2023b. GPR’s ability to handle small datasets, flexibility in capturing non-linear relationships, and suitability for sequential learning make it advantageous in this context. The dataset was divided into a ratio of 80:20 for training and testing, respectively. This partition facilitated the assessment of the model’s generalization capability, leading to the creation of a robust soft sensor capable of predicting methanol syn thesis performance in diverse conditions.
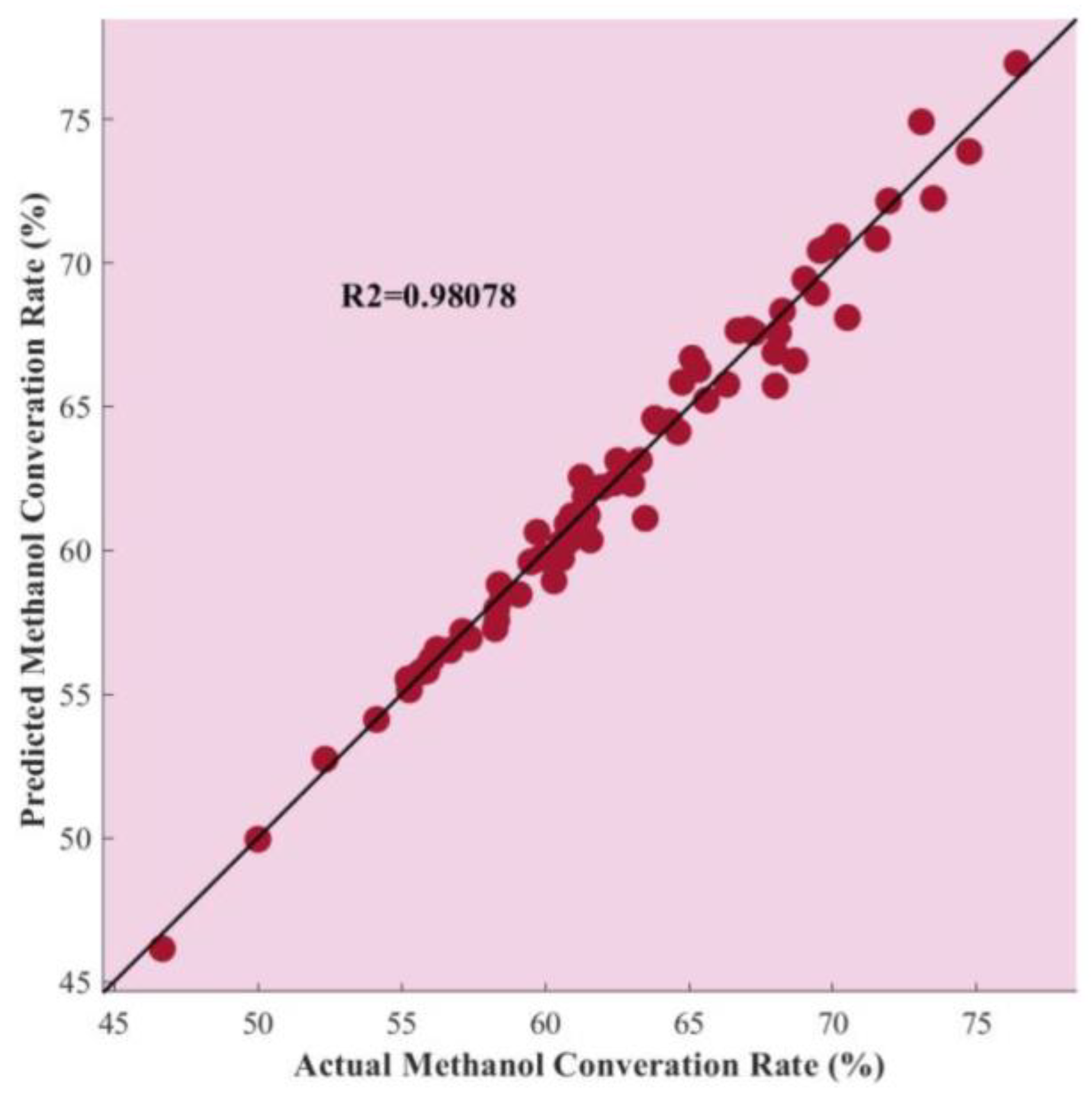
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R. Global Warming of 1.5 C: IPCC Special Report on Impacts of Global Warming of 1.5 C above Pre-Industrial Levels in Context of Strengthening Response to Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Hasan, M.F.; Zantye, M.S.; Kazi, M.-K. Challenges and opportunities in carbon capture, utilization and storage: A process systems engineering perspective. Comput. Chem. Eng. 2022, 166, 107925. [Google Scholar] [CrossRef]
- Yusuf, N.; Almomani, F. Highly effective hydrogenation of CO2 to methanol over Cu/ZnO/Al2O3 catalyst: A process economy & environmental aspects. Fuel 2023, 332, 126027. [Google Scholar]
- Alsayegh, S.; Johnson, J.; Ohs, B.; Wessling, M. Methanol production via direct carbon dioxide hydrogenation using hydrogen from photocatalytic water splitting: Process development and techno-economic analysis. J. Clean. Prod. 2019, 208, 1446–1458. [Google Scholar] [CrossRef]
- Montebelli, A.; Visconti, C.G.; Groppi, G.; Tronconi, E.; Ferreira, C.; Kohler, S. Enabling small-scale methanol synthesis reactors through the adoption of highly conductive structured catalysts. Catal. Today 2013, 215, 176–185. [Google Scholar] [CrossRef]
- Ahmad, I.; Ayub, A.; Ibrahim, U.; Khattak, M.K.; Kano, M. Data-based sensing and stochastic analysis of biodiesel production process. Energies 2018, 12, 63. [Google Scholar] [CrossRef]
- Khan, J.S.; Ahmad, I.; Jadoon, U.K.; Samad, A.; Saghir, H.; Kano, M.; Caliskan, H. Artificial intelligence based prediction of optimum operating conditions of a plate and fin heat exchanger under uncertainty: A gray-box approach. Int. J. Heat Mass Transf. 2023, 217, 124653. [Google Scholar] [CrossRef]
- Samad, A.; Ahmad, I. An Intelligent System for Estimation of Exergy Efficiency of Integrated Naphtha and Isomerization Process under Uncertainty. In Proceedings of the 2022 17th International Conference on Emerging Technologies (ICET), Swabi, Pakistan, 29–30 November 2022; pp. 12–17. [Google Scholar]
- Samad, A.; Ahmad, I.; Kano, M.; Caliskan, H. Prediction and optimization of exergetic efficiency of reactive units of a petroleum refinery under uncertainty through artificial neural network-based surrogate modeling. Process Saf. Environ. Prot. 2023, 177, 1403–1414. [Google Scholar] [CrossRef]
- Bussche, K.V.; Froment, G.F. A steady-state kinetic model for methanol synthesis and the water gas shift reaction on a commercial Cu/ZnO/Al2O3Catalyst. J. Catal. 1996, 161, 1–10. [Google Scholar] [CrossRef]
- Mignard, D.; Pritchard, C. On the use of electrolytic hydrogen from variable renewable energies for the enhanced conversion of biomass to fuels. Chem. Eng. Res. Des. 2008, 86, 473–487. [Google Scholar] [CrossRef]
- Van-Dal, É.S.; Bouallou, C. Design and simulation of a methanol production plant from CO2 hydrogenation. J. Clean. Prod. 2013, 57, 38–45. [Google Scholar] [CrossRef]
| Data Samples | Molar Flowrate (kmol/h) | Temperature (°C) | Pressure (kPa) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | H2 | 5.00 | 6.00 | 14.00 | 20.00 | CO2 | H2 | 1.00 | 2.00 | 5.00 | 9.00 | 10.00 | 12.00 | 19.00 | CO2 | H2 | Conversion | |
| 1.00 | 76.46 | 535.22 | 210.00 | 284.00 | 35.00 | 42.37 | 80.00 | 12.30 | 7800.00 | 7800.00 | 7570.00 | 7480.00 | 7360.00 | 7360.00 | 7800.00 | 4763.00 | 3000.00 | 61.94 |
| 2.00 | 78.87 | 556.94 | 202.17 | 295.74 | 35.46 | 38.40 | 12.36 | 26.14 | 8162.61 | 7532.94 | 7926.24 | 7821.96 | 7349.24 | 7581.01 | 7520.67 | 4961.01 | 3087.66 | 58.83 |
| 3.00 | 82.49 | 565.61 | 192.78 | 306.07 | 37.00 | 35.15 | 12.66 | 25.86 | 8289.52 | 7285.25 | 8089.56 | 7455.76 | 7185.29 | 7236.96 | 7217.69 | 5057.67 | 3031.19 | 54.50 |
| 4.00 | 84.83 | 543.88 | 186.31 | 310.04 | 37.05 | 34.84 | 13.14 | 26.90 | 8450.52 | 7168.99 | 8004.65 | 7112.03 | 7563.63 | 7168.62 | 7193.36 | 5273.91 | 3122.98 | 51.24 |
| 5.00 | 74.14 | 521.90 | 212.44 | 283.24 | 34.48 | 36.30 | 12.36 | 26.04 | 7632.95 | 8000.62 | 7762.07 | 7390.57 | 7409.92 | 7047.83 | 7452.08 | 4895.97 | 3130.20 | 63.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samad, A.; Saghir, H.; Musawwir, A.; Zulkefal, M. An Intelligent System for Predicting the Methanol Conversion Rate from the Direct Hydrogenation of CO2 under Uncertainty. Mater. Proc. 2024, 17, 3. https://doi.org/10.3390/materproc2024017003
Samad A, Saghir H, Musawwir A, Zulkefal M. An Intelligent System for Predicting the Methanol Conversion Rate from the Direct Hydrogenation of CO2 under Uncertainty. Materials Proceedings. 2024; 17(1):3. https://doi.org/10.3390/materproc2024017003
Chicago/Turabian StyleSamad, Abdul, Husnain Saghir, Abdul Musawwir, and Muhammad Zulkefal. 2024. "An Intelligent System for Predicting the Methanol Conversion Rate from the Direct Hydrogenation of CO2 under Uncertainty" Materials Proceedings 17, no. 1: 3. https://doi.org/10.3390/materproc2024017003
APA StyleSamad, A., Saghir, H., Musawwir, A., & Zulkefal, M. (2024). An Intelligent System for Predicting the Methanol Conversion Rate from the Direct Hydrogenation of CO2 under Uncertainty. Materials Proceedings, 17(1), 3. https://doi.org/10.3390/materproc2024017003







