Exergy Analysis of an Alkaline Water Electrolysis System †
Abstract
1. Introduction
2. Materials and Methods
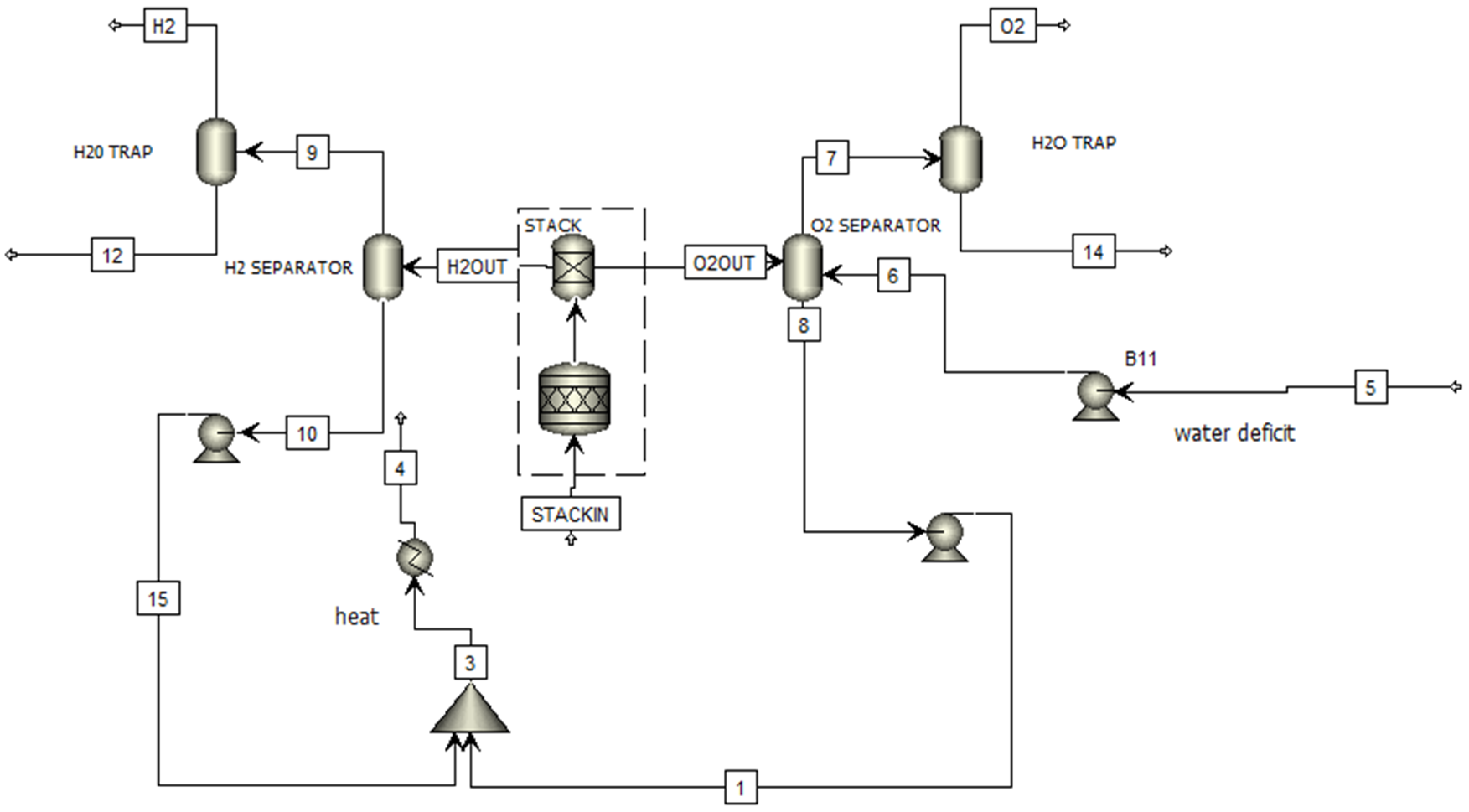
2.1. Process Description
2.2. Exergy Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charvin, P.; Stéphane, A.; Florent, L.; Gilles, F. Analysis of solar chemical processes for hydrogen production from water splitting thermochemical cycles. Energy Convers. Manag. 2008, 49, 1547–1556. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Sanchez, M.; Amores, E.; Abad, D.; Rodriguez, L.; Clemente-Jul, C. Aspen Plus model of an alkaline electrolysis system for hydrogen production. Int. J. Hydrogen Energy 2020, 45, 3916–3929. [Google Scholar] [CrossRef]
- Zhang, H.; Su, S.; Lin, G.; Chen, J. Configuration designs and parametric optimum criteria of an alkaline water electrolyzer system for hydrogen production. Int. J. Electrochem. Sci 2011, 6, 2566–2580. [Google Scholar] [CrossRef]
- Hammoudi, M.; Henao, C.; Agbossou, K.; Dubé, Y.; Doumbia, M.L. New multi-physics approach for modelling and design of alkaline electrolyzers. Int. J. Hydrogen Energy 2012, 37, 13895–13913. [Google Scholar] [CrossRef]
- Mustafa, J.; Ahmad, I.; Ahsan, M.; Kano, M. Computational fluid dynamics based model development and exergy analysis of naphtha reforming reactors. Int. J. Exergy 2017, 24, 344–363. [Google Scholar] [CrossRef]
- Samad, A.; Ahmad, I.; Kano, M.; Caliskan, H. Prediction and optimization of exergetic efficiency of reactive units of a petroleum refinery under uncertainty through artificial neural network-based surrogate modeling. Process Saf. Environ. Prot. 2023, 177, 1403–1414. [Google Scholar] [CrossRef]
- Samad, A.; Saghir, H.; Ahmad, I.; Ahmad, F.; Caliskan, H. Thermodynamic analysis of cumene production plant for identification of energy recovery potentials. Energy 2023, 270, 126840. [Google Scholar] [CrossRef]
| Equipment | Exergy Destruction (KW) | Exergy Efficiency (%) | Improvement Potential (KW) |
|---|---|---|---|
| Stack | 9.20532 | 24.64893 | 6.936305322 |
| H2 Separator | 0.00031 | 99.98000 | 0.000000061 |
| H2O Trap 1 | 0.07978 | 34.72741 | 0.052073033 |
| Pump 1 | 0.00123 | 99.91311 | 0.000001071 |
| Mixer 1 | 0.20000 | 92.93972 | 0.014120266 |
| Heater | 0.16570 | 90.00552 | 0.016560510 |
| O2 Separator | 0.00151 | 99.89795 | 0.000001536 |
| H2O Trap 2 | 0.07978 | 34.72741 | 0.052073033 |
| Pump 2 | 0.00009 | 76.14504 | 0.000022647 |
| Pump 3 | 0.00124 | 99.91272 | 0.000001080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sethi, H.; Zulkefal, M.; Ayub, A. Exergy Analysis of an Alkaline Water Electrolysis System. Mater. Proc. 2024, 17, 13. https://doi.org/10.3390/materproc2024017013
Sethi H, Zulkefal M, Ayub A. Exergy Analysis of an Alkaline Water Electrolysis System. Materials Proceedings. 2024; 17(1):13. https://doi.org/10.3390/materproc2024017013
Chicago/Turabian StyleSethi, Hamza, Muhammad Zulkefal, and Asad Ayub. 2024. "Exergy Analysis of an Alkaline Water Electrolysis System" Materials Proceedings 17, no. 1: 13. https://doi.org/10.3390/materproc2024017013
APA StyleSethi, H., Zulkefal, M., & Ayub, A. (2024). Exergy Analysis of an Alkaline Water Electrolysis System. Materials Proceedings, 17(1), 13. https://doi.org/10.3390/materproc2024017013







