1. Introduction
Clathrate hydrates, also known as gas hydrates, form when gas molecules are trapped within a lattice-like structure of water molecules under specific pressure and temperature conditions [
1]. These structures consist of cages formed by water molecules, with gas molecules occupying the cavities of the lattice [
2]. Clathrate hydrates can encapsulate various gases, such as methane, carbon dioxide, nitrogen, and hydrogen [
3]. They are commonly found in natural environments like deep-sea sediments and permafrost regions [
4].
The study of clathrate hydrates is highly important in various fields, including energy production and storage, climate change, and gas transportation [
5]. Methane hydrates hold great potential as a substantial energy source due to their significant methane content [
6]. Exploring the extraction and utilization of methane hydrates is an area of active interest. Furthermore, clathrate hydrates have exceptionally high volumetric gas storage capacity, making them a promising solution for technological applications such as hydrogen and natural gas storage, as well as carbon dioxide storage [
4,
7]. However, the release of greenhouse gases from melting hydrates can contribute to global warming, raising concerns about their stability and the potential impact on climate change [
8]. Moreover, hydrate formation in oil and gas pipelines can lead to flow blockages and operational challenges, posing risks to safety, health, and the environment [
5]. Therefore, accurately predicting hydrate stability conditions is crucial for industries involved in these sectors.
Efforts have been focused on developing reliable prediction tools for determining hydrate stability conditions and phase diagrams in single and multi-component systems, considering the presence or absence of inhibitors. Various methodologies have been employed, including the K-value method, gas gravity diagrams, and rigorous thermodynamic models. The K-value method, pioneered by Katz and Wilcox, involves generating diagrams to describe equilibrium among hydrocarbons, water, and hydrates [
9]. Gas gravity diagrams provide a simplified representation of hydrate stability but may lack accuracy for hydrocarbon mixtures, especially when non-hydrocarbon gas components are present [
10]. Rigorous thermodynamic modeling, on the other hand, is considered the most effective approach for accurate predictions. It incorporates equations of state (EoS) and phase equilibrium calculations, taking into account the properties and interactions of each component.
The foundation of thermodynamic modeling for hydrates can be traced back to the work of Van der Waals and Platteeuw in 1958, where they derived a statistical thermodynamic equation for gas hydrates based on the Lennard-Jones potential [
11]. This model focused on the solid phase and has since been typically combined with an equation of state and an activity coefficient model to describe co-existing fluid phases. A subsequent study by McKoy and Sinanoğlu in 1963 improved predictions of hydrate dissociation conditions by introducing cell potentials like the Kihara one [
12]. Parrish and Prausnitz, in 1972, further modified the VdW-P approach, making it suitable for computer calculations of hydrate phase equilibria [
13]. Currently, commercial PVT software packages such as Multiflash, HydraFLASH, CSMGem, and CSMHyd utilize different computational methodologies, including hydrate modeling (original vs. modified VdW-P), equation of state (EoS) models (cubic vs. cubic-plus-association EoS), thermodynamic models (Debye–Huckel with/without additional terms), and phase behavior representation (activity coefficient vs. EoS), to predict hydrate formation conditions rigorously. These software packages vary in their approaches, which can impact the accuracy of predicting hydrate dissociation conditions.
To address this variability and assess their performance, it is crucial to evaluate these software packages using diverse systems, including complex multi-component gas hydrate former systems and highly inhibited systems. Additionally, an extensive database of experimental dissociation pressure data should be considered. In this study, we comprehensively evaluate four commercial simulators (Multiflash by KBC, HydraFLASH by Hydrafact, and CSMGem and CSMHyd by E. Sloan) by considering five thermodynamic models adopted within these simulators. While these software packages undergo regular tuning and verification, our focus is to compare their performance against complex systems using independent experimental data retrieved from published papers between 2015 and 2019 that has not been used in their tuning process at the time of the assessment. By subjecting the models to their limits, we aim to provide a fair and thorough comparison of their predictive capabilities, ensuring a robust evaluation of the simulators’ performance.
2. Software Packages—Thermodynamic Models
The four commercial software packages for phase equilibrium evaluated in this work are summarized in
Table 1. In addition, the five thermodynamic models used within these packages are discussed in this section.
2.1. HydraFLASH CPA (HF CPA)
HydraFLASH is a software package that offers a comprehensive solution for modeling hydrate systems. It determines the most stable hydrate structure (sI, sII, or sH) in equilibrium for a given water/hydrocarbon/inhibitor mixture and incorporates the inhibition effect of salts/alcohols, allowing the estimation of the minimum inhibitor injection rate required to prevent hydrate formation. The software employs Michelsen’s multiphase flash algorithm and combines the Van der Waals–Platteeuw (VdW-P) theory with the CPA (cubic-plus-association) equation of state (EoS) to implement a fugacity-based model. In the HF CPA model, the non-associative term utilizes the Soave–Redlich–Kwong (SRK) and Peng–Robinson (PR) cubic equations of state. To ensure a fair comparison with other CPA models (MF CPA, discussed below), the SRK-EoS was selected as the non-associative part of the CPA model, supported by the existing literature and previous successful experiences in modeling hydrate systems, particularly when considering the inhibitor effects [
14,
15]. The Soave model was chosen for the attractive term due to its ability to provide consistent predictions of saturation properties, enthalpies, and heat capacities. Additionally, the Huron–Vidal mixing rule was employed to accurately capture the energetic effects and asymmetry present in both simple and complex mixtures [
16]. In the modeling of electrolyte solutions, HydraFLASH incorporates the Debye–Hückel term into the total residual Helmholtz free energy. In addition, the Poynting factor is utilized to correct the fugacity of water and accurately model the fugacity of the ice phase.
2.2. Multiflash CPA (MF CPA) and Multiflash RKSA (MF RKSA)
Multiflash, developed by KBC, is another comprehensive software package specifically designed for modeling hydrate systems. It offers two distinct models: MF CPA (Multiflash cubic-plus-association) and MF RKSA (Multiflash Redlich–Kwong–Soave Advanced), each providing advanced capabilities to predict hydrate dissociation/formation conditions, quantify hydrate amounts, identify gas hydrate equilibrium types (sI, sII, and sH), and simulate phase transitions between the fluid and hydrate phases. Additionally, Multiflash supports the modeling of inhibition effects, enabling users to assess the impact of inhibitors on hydrate stability. The software also facilitates the estimation of the required inhibitor injection rate, aiding in the design of effective mitigation strategies to prevent hydrate formation.
The MF CPA model in Multiflash incorporates the cubic-plus-association equation of state and utilizes the original VdW-P theory. It employs the SRK equation of state as the non-associative term and employs polynomial functions to describe the alpha parameter. The model also utilizes standard Van der Waals mixing rules in conjunction with the generic numerical formulation of CPA. To account for the electrolysis effects of aqueous salt solutions, the Debye–Huckel method is adopted, followed by virial corrections and a Born term [
17,
18]. On the other hand, the MF RKSA model in Multiflash utilizes an advanced version of the Redlich–Kwong–Soave equation of state. It combines the RKS or SRK equation of state with an NRTL (non-random two-liquid)-type mixing rule to handle phase equilibrium calculations. In contrast to the MF CPA model, the MF RKSA model considers only the residual Helmholtz free energy of the system as a function of the SRK term, without incorporating additional association or electrolyte terms. For electrolytes, MF RKSA employs the pseudo-salt component approach, assuming that salts are represented by a single pseudo-NaCl-equivalent salt component. This component possesses its own physical properties and binary interaction parameters, behaving as a non-volatile heavy component in the aqueous phase. It does not interfere with the hydrocarbon phase and precipitates at the solubility limit. Therefore, when dealing with aqueous-phase solutions containing various types of salts (e.g., MgCl
2, CaCl
2, NaCl), user-defined rules are assigned to convert the mixed salt solution into a single equivalent pseudo-NaCl solution. Both the MF CPA and MF RKSA models utilize the freeze-out model to calculate phase equilibrium and the properties of other solid phases that may coexist with hydrates, such as ice.
2.3. CSMGem (CSMGem) and CSMHyd (CSMHyd)
CSMGem, developed at the Center of Hydrate Research at the Colorado School of Mines (CSM), is a software package specifically designed for hydrate modeling. It incorporates the modified Van der Waals–Platteeuw model as a fugacity-based approach, coupled with the Soave–Redlich–Kwong equation of state to model hydrocarbon phases. For the aqueous phase, CSMGem utilizes the Shock–Helgeson equation and the Bromley activity model. The software employs the flash algorithm proposed by Gupta et al. [
19], which is based on Gibbs energy minimization, to perform multi-phase equilibria calculations. Meanwhile, CSMGem has the capability to handle equilibrium calculations involving up to 10 phases, depending on the composition of the feed. This includes vapor and liquid hydrocarbon phases, an aqueous phase, ice, gas hydrates (sI, sII, and sH structures), as well as solid phases of NaCl, KCl, and CaCl
2 (MgCl
2 is not supported). The software employs an iterative approach in the flash algorithm to determine the number of phases present, reducing them throughout the iterations. In addition, CSMGem provides detailed information regarding the phases present, hydrate structures, cage occupancy in specific pressure and temperature conditions, and the compositions of the equilibrium phases.
On the other hand, CSMHyd, the predecessor of CSMGem, focuses on predicting the formation temperature and pressure of sI and sII hydrate structures. It can also consider the presence of methanol and/or salts and handle three-phase or four-phase conditions. CSMHyd supports eight salts, namely NaCl, Na2SO4, NaF, KBr, KCl, CaCl2, MgCl2, and SrCl2. It utilizes the original VdW-P approach coupled with the SRK EoS and a variation of the P&P method. The software outputs information about the incipient hydrate phase, including equilibrium phases (liquid water–hydrate–vapor, ice–hydrate–vapor, or liquid water–hydrate–vapor–liquid hydrocarbon), hydrate structure type, hydrate dissociation/formation conditions, phase compositions, and hydrate cage fractional occupancy. Similar to CSMGem, CSMHyd utilizes the SRK EoS coupled with a statistical thermodynamic model to predict hydrate dissociation conditions. It employs a fugacity-based approach to estimate equilibrium conditions of pressure and temperature, aiming to match the theoretical and experimental values.
3. Experimental Data and Result Visualization
3.1. Experimental Database
The experimental database for hydrate dissociation points was constructed by gathering recent data from 17 journal papers published between 2015 and 2019. These papers encompassed information on hydrate dissociation pressure and temperature for various gas stream compositions. To ensure a systematic evaluation, the collected data points were categorized into two groups: uninhibited systems and inhibited systems.
Within the uninhibited systems group, further divisions were made based on the characteristics of the systems. The data points were organized into two collections: one focusing on flow assurance issues and the other on industrial applications. Consequently, a total of four uninhibited systems were identified. In the inhibited group, the systems involved methane hydrate formers with different salt types and concentrations. This group was divided into two collections based on the presence or absence of salts in the aqueous phase, resulting in an additional five systems.
By adopting this categorization, a well-structured and organized evaluation process was established, facilitating the analysis and comparison of hydrate dissociation points.
Table 2 presents the generated hydrate dissociation database, outlining the relevant systems examined.
3.2. Result Analysis and Visualization
The performance of the evaluated software packages was comprehensively assessed using various metrics, allowing for a thorough evaluation of their performance. The metrics considered included the absolute error range, as well as the average signed and absolute pressure errors within each group/collection, and their respective deviations. However, in this work, a detailed presentation and discussion of these metrics are omitted, as they require in-depth reporting and analysis. Instead, a simplified comparison is provided by focusing on important statistics, namely the average pressure error and pressure standard deviation . These statistics provide a concise summary of the software packages’ performance. By evaluating the average behavior of each software package through comparative plots that display the values, a clearer understanding of the variations in their performance can be obtained. These plots facilitate the visual comparison of the software packages and highlight their relative strengths and weaknesses.
Furthermore, an overall evaluation was attempted using spider plots, which provide a comprehensive assessment of the software packages’ performance. Spider plots allow for a holistic view of performance across multiple systems, enabling a more comprehensive understanding of the mean absolute error (MAE) and mean error (ME) per system and per software. The MAE is calculated as the average of the absolute errors, while the ME is calculated as the average of the errors as follows:
where
is the experimental pressure and
is the calculated pressure for point
using thermodynamic model
, whereas MAE and ME are calculated by:
where
is the number of data points in the system evaluated.
Through these analysis techniques and visualizations, a comprehensive evaluation of the software packages’ performance was achieved, providing insights into their accuracy and reliability in predicting hydrate dissociation conditions.
4. Results
To evaluate the average behavior of each software package, comparative plots were generated, displaying the
values. In
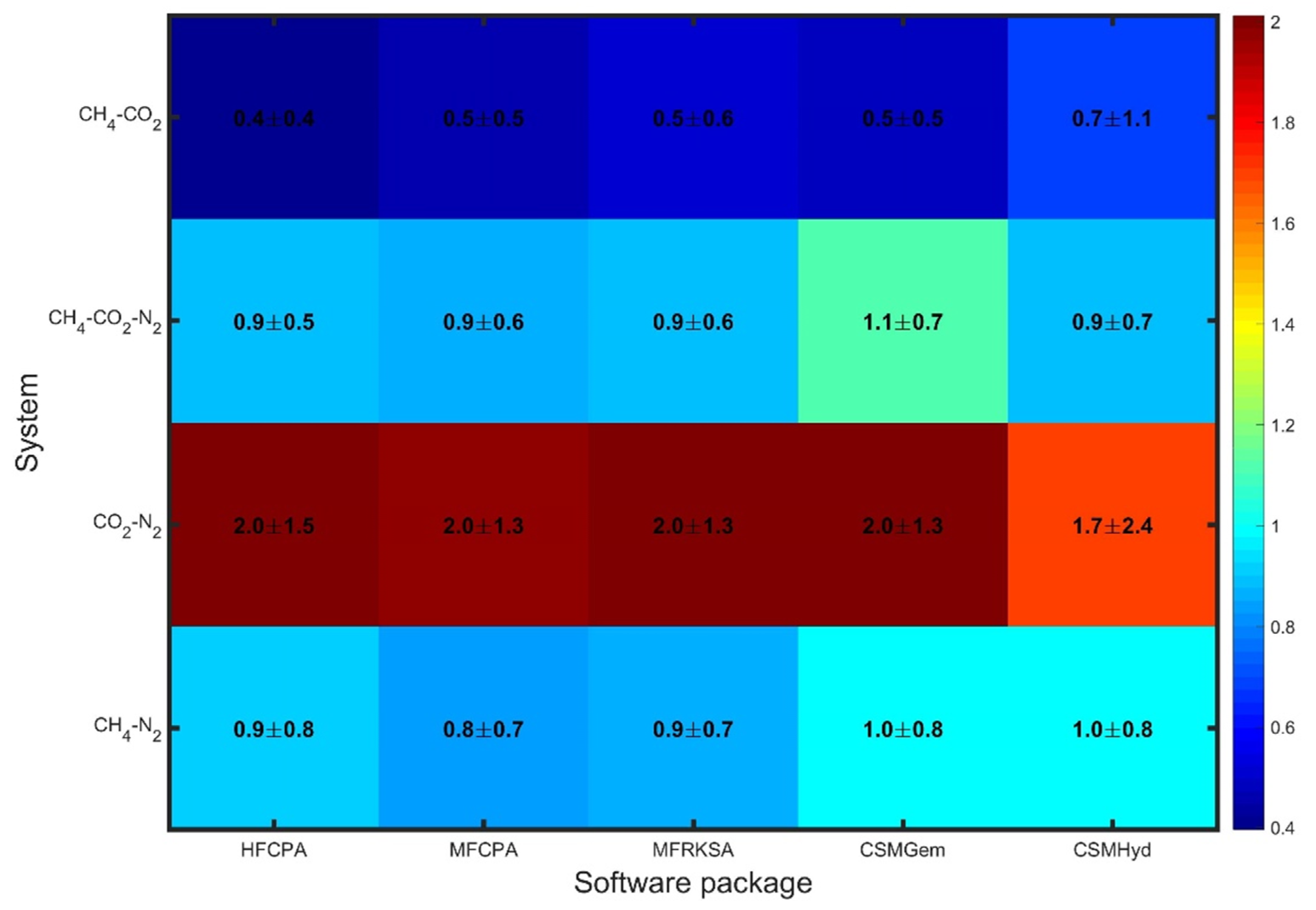
Figure 1, the errors obtained for all uninhibited systems are presented, revealing significantly varying performance among the tested software packages. For CH
4-CO
2 mixtures, all the models predict hydrate dissociation conditions accurately, with only slight deviations (<0.7 Mpa) that can be considered affordable for handling flow assurance issues. Similarly, the CH
4-CO
2-N
2 and CH
4-N
2 systems exhibit slightly enhanced errors, which are still within an acceptable range. The maximum average absolute error in these cases does not exceed 1 MPa (145 psi). However, all approaches exhibit weak performance when it comes to CO
2-N
2 mixtures, with errors equal to or above 1.7 MPa. This indicates a challenge in accurately predicting hydrate dissociation conditions for such mixtures. Overall, the software packages show similar performance for each individual system, as indicated by the similar colors in each row of the comparative plot. This suggests uniform prediction capability among the available modeling techniques for uninhibited systems.
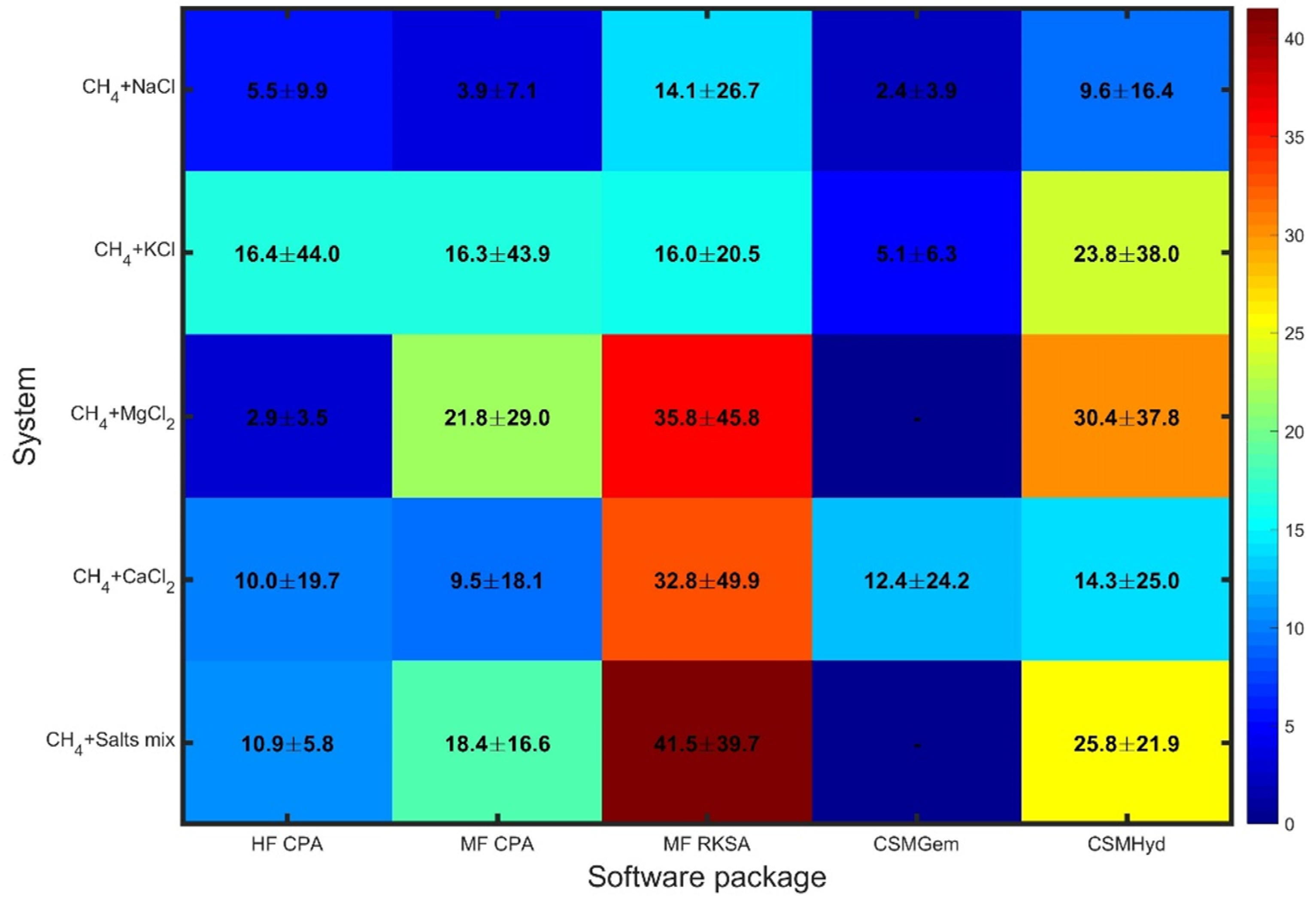
Figure 2 reveals a different pattern when considering inhibited systems, with similarities observed across the software packages (similar colors in each column) rather than across the different systems. The errors in the predictions not only show a significant enhancement but also exhibit considerable variation among the examined systems.
CSMGem stands out as it provides predictions of superior accuracy compared to the other packages for the systems it is capable of handling (excluding MgCl2). Its performance is noteworthy, indicating its reliability in predicting dissociation pressures for inhibited systems.
On the other hand, MF RKSA shows notably large deviations, particularly for systems containing MgCl2, CaCl2, and salt mixtures. This highlights the weakness of the pseudo-salt NaCl-equivalent component approach and the use of SRK combined with NRTL in this package. The limitations of MF RKSA in accurately capturing the thermodynamics of these systems are evident. CSMHyd performs better than MF RKSA, although it still yields highly deviating predictions for most of the inhibited systems examined. While it shows an improvement in performance, there is room for further enhancement in its accuracy. In contrast, both models based on the CPA approach demonstrate decent performance across all systems, offering a reliable solution for predicting dissociation pressures in inhibited systems containing pure salts. HF CPA outperforms MF CPA in methane systems with salt mixtures, which are commonly encountered in natural gas production. The effectiveness of the CPA methodology in capturing the complex thermodynamics of these systems is evident in their performance. Overall, these results emphasize the importance of selecting an appropriate software package for accurate predictions in inhibited systems. CSMGem proves to be a favorable choice, while the CPA-based models provide reliable predictions across all inhibited systems, demonstrating their robustness and effectiveness. These findings underscore the significance of considering the software package’s capabilities and methodologies when aiming for accurate predictions in inhibited systems.
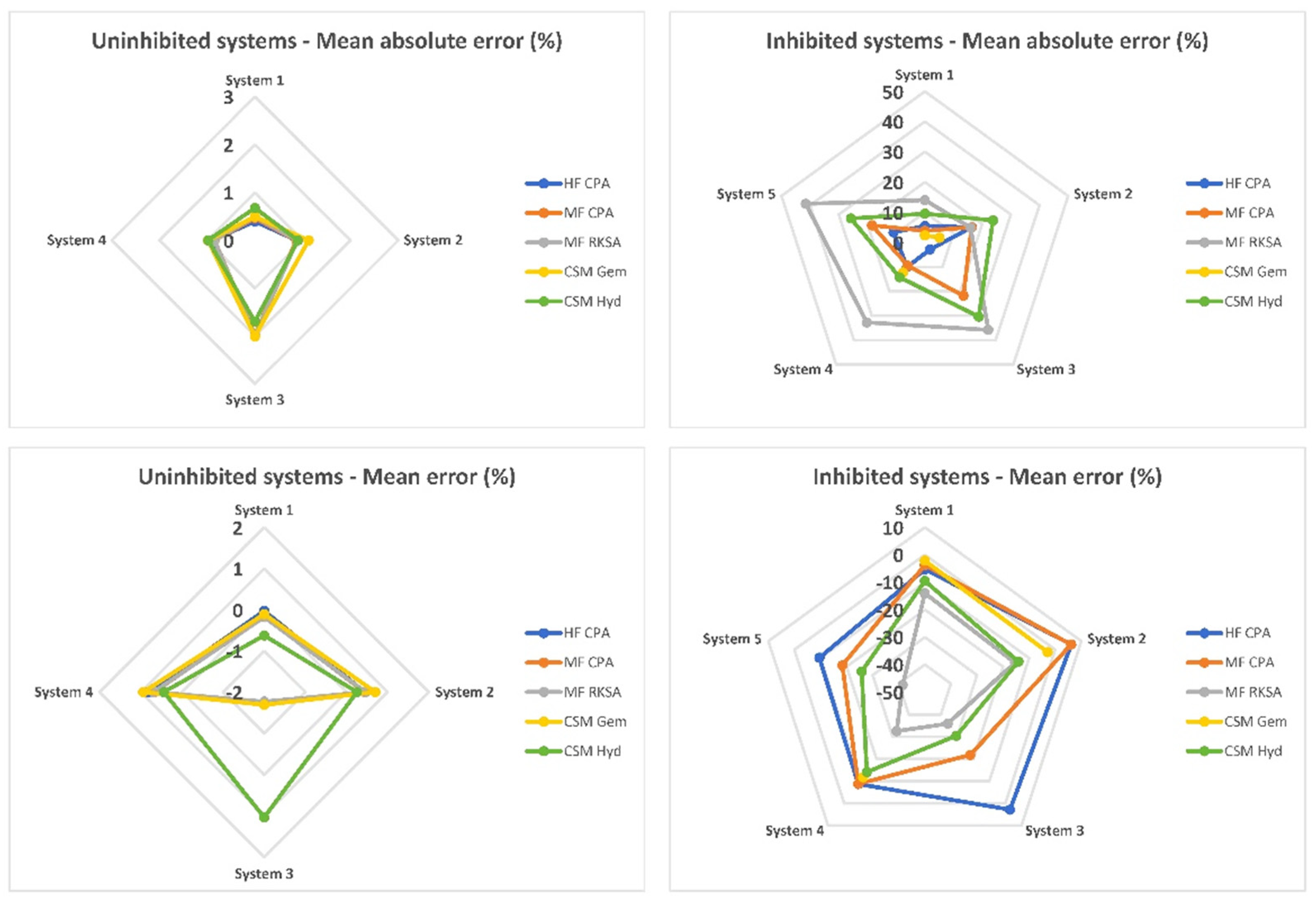
An overall evaluation of the software packages can be conducted using spider plots, as depicted in
Figure 3. In classic, uninhibited systems, the performance of all software packages is quite similar, with minimal bias and average absolute errors barely exceeding 2 MPa. This indicates that there are no clear winners among the tested packages for these types of systems. However, when it comes to inhibited systems, a notable difference in performance is observed. MF RKSA and CSMHyd exhibit severe errors in their predictions for inhibited systems, indicating limitations in accurately capturing the thermodynamics of these systems. On the other hand, HF CPA and MF CPA perform much better, showing improved accuracy in predicting dissociation pressures. Among these two, HF CPA stands out as the leading performer due to its lower and better-balanced average error. This indicates its reliability and accuracy in predicting dissociation pressures for inhibited systems. HF CPA demonstrates robust performance across a range of inhibited systems, making it a preferred choice for accurate predictions. While CSMGem demonstrates optimal and well-balanced performance for inhibited systems, it is important to note its limitation in handling systems inhibited by MgCl
2. This limitation may restrict its usability in scenarios involving that specific salt. Therefore, when considering inhibited systems, HF CPA emerges as the preferred choice due to its superior and well-balanced performance. However, it is necessary to carefully evaluate the specific requirements of the system and the capability of the software package to handle the inhibitors involved before making a final selection.
5. Conclusions
In this study, five commercial software packages (HF CPA, MF CPA, MF RKSA, CSMGem, and CSMHyd) were evaluated and compared regarding their prediction of hydrate dissociation conditions in both inhibited and uninhibited systems. The comparison was based on a large experimental database consisting of 400 data points.
The results of this study indicate that all five models provide reliable and balanced predictions of dissociation pressure for uninhibited flow assurance systems. The average errors obtained for the CH4-CO2 and CH4-CO2-N2 mixtures were within an acceptable range, indicating accurate predictions that are suitable for handling flow assurance issues. For systems containing CO2-N2 and CH4-N2, the prediction accuracy of all models, except for CSMHyd, was considered sufficiently reliable for most technological applications. However, these systems showed higher sensitivity to the N2 component, which affected the accuracy of the predictions.
In the case of inhibited systems with monovalent cations such as NaCl and KCl, CSMGem exhibited remarkably balanced accuracy compared to the other models. The CPA-based models, MF CPA and HF CPA, were ranked as the second-best predictors for these systems. For systems with divalent cations, like MgCl2 and CaCl2, HF CPA, provided remarkably accurate predictions for both salts, while MF CPA performed accurately only for CaCl2. It should be noted that MF RKSA and CSMHyd showed poor predictive performance for systems with divalent cations, indicating limitations in accurately predicting dissociation pressure values for flow assurance or other hydrate applications.
In the group of methane hydrate-forming systems with salt mixtures, which is commonly encountered in dry gas production streams, CPA-based methods, particularly HF CPA, demonstrated the best performance, providing the most accurate predictions.
Overall, while all software packages exhibited similar performance for uninhibited systems, they showed distinct performance profiles with individual strengths and weaknesses when it came to inhibited systems. HF CPA emerged as the most accurate and balanced tool for predicting dissociation pressure across different system types, followed closely by MF CPA. The other models, CSMGem, CSMHyd, and MF RKSA, exhibited varying levels of accuracy.