Abstract
We tested some possible processes for using ashes from incinerators, both fly and bottom ash, and included older processes, like the Schnabel process, in our assessment. The possibilities for selective leaching with ammonia and ammonium carbonate are utilized, as well as ammonia recycling. Fly ash and bottom ash may be treated similarly but with some specific differences. Our conclusion is that recovery from incinerated municipal waste ash is an economical and viable source of copper and zinc. Such a process will also reduce the amount of waste to handle and make it more chemically stable due to the alkalinity of the residue.
1. Introduction
The EU’s 2020 list of critical raw materials (CRM) comprises more than 40 elements, and the EU’s CRM Act sets a goal of at least 10% of each of these materials to be produced in Europe and at least 15% to be recycled. Ashes from incinerated municipal waste are known to contain critical metals such as copper and antimony, in addition to important base metals like zinc and tin. Our work has shown that the potential recovery of copper and zinc may be in the same order of magnitude as virgin ores. Such source materials are ready to be treated hydrometallurgically compared to primary production. Costly mining, grinding, milling and beneficiation operations of ores are avoided. However, the heterogeneity of the contents in municipal waste to be incinerated is significant since it will vary with factors like time and consumer trends, the site where the waste is collected and the incineration technology that is used.
Municipal waste volumes in the Western world are huge. Normally, municipal waste is collected and incinerated in order to reduce volumes and recover energy. This creates energy for consumption and causes reduced volumes to deposit and handle. For example, Norway’s five million inhabitants generate 1.5 million tons to be incinerated annually. Nedkvitne et al. [1] provided an overview of the incinerated waste in Northern Europe in 2020 and noted the annual increase in incinerated waste. Such incineration creates two types of waste to be handled: bottom ash and fly ash, including air pollution control residues.
As the line between fly ash and APC residue is not always very clear in the literature, we will use fly ash as a collective term in this article. Both components can be divided into different types after the technology used for the incineration. Still, in our work, we have strived to develop a robust process capable of handling all kinds of incinerated waste, and we have focused on the constituents of the highest values.
The content in municipal waste will vary over time and also depend on the region it is collected from. Therefore, the value of what is in the waste will vary. However, the most valuable constituents are copper, zinc and lead. These metals are, on average, so high in concentration that these ashes may not be deposited at landfills due to regulations concerning the content of toxic pollutants. Based on 895 XRF analyses of fly ash, Nedkvitne et al. (2021) [2] showed that, on average, Zn is about 10, Cu 1.2, V, Cd, and Ni 0.2 g per kg fly ash, respectively. The variations are, however, huge. The most abundant components are Ca, Cl, Na, Si, S, K, Al and Fe. In Figure 1, the averages of some selected elements found in bottom ash in the EU are shown. The concentration of each element is the calculated median of the data gathered, and the number of samples for each element varies between 50 and 1700. The major elements (>10 g/kg) are shown in the left panel, while the minor elements (>1 g/kg) are shown in the right panel. Trace elements (<1 g/kg) are not shown in the figure. Nedkvitne et al. (2023) [2] have also shown that the copper and zinc content in the ashes in question is the same magnitude as that of low-concentrated mineral ores. Compared to ore, there is no need for mining and no gangue material to address. These three metals, Cu, Zn and Pb, are found in both fly ash and bottom ash, but the two former metals are abundant while the concentration of Pb is mostly quite low.
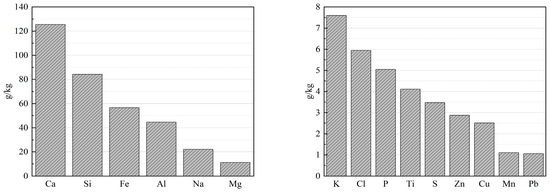
Figure 1.
Overview of the average elemental composition of municipal solid waste bottom ash based on data gathered from individual member states of the European Union (EU). Left panel: Major constituents; Right panel: Minor constituents, but with concentrations >1 g/kg. The data were made available by the Confederation of European Waste-to-Energy Plants (CEWEP) [3].
Copper and zinc are both considered to be soft Lewis acids together with nickel, cadmium, mercury, silver, etc. [4]. To extract Cu and Zn selectively from oxidizing incineration ashes, a soft Lewis base is needed. One type of leaching agent is appealing: ammonia (solution). This makes the pregnant leach solution (PLS) alkaline, meaning that most metals with low soluble hydroxides (harder Lewis acids) will not enter the solution. Both copper and zinc form ammine complexes that will keep them in solution and increase their solubility. In addition, ammonia can be recycled by heating, so the consumption of ammonia can be kept to a minimum, and alkaline solutions are usually less corrosive than acidic ones.
Ammoniacal leaching is a well-known topic. A useful tool to see at which pH and redox-potential the various metals of interest are in solution and what kind of species is the Pourbaix or Eh-pH diagram. Meng and Han [5] provided such diagrams for both Cu and Zn. However, they only included one kind of ammonia complex for Cu(II) and one for Cu(I). Wei et al. [6], in a very comprehensive study of the leaching of copper oxide with ammoniacal solutions, claimed the presence of five complexes, all with quite large complexing constants, βi:
Here, the bracket parentheses denote activity. According to the Pourbaix diagram provided by Meng and Han [5] the pH for leaching should be in the range 8–10 in order to leach both Cu(II) and Zn(II). A pure ammoniacal solution will have a pH of almost 12. To reduce the pH to the optimum interval, ammonium is the preferred acid, which forms a buffer with ammonia. Liu et al. [5] preferred NH4Cl but had to include the low solubility of the compound Cu(OH)1.5Cl0.5 and also other combinations of copper compounds with ammonia, chloride and hydroxide. Bingöl et al. [7] argued for the use of (NH4)2CO3 for the leaching of CuO, and Rodriguez et al. [8] did the same for ZnO. Such solutions consisting of ammonia and ammonium carbonate are denoted AAC. Recovering zinc by using AAC has been known since 1880, and the process is named after the inventor, Schnabel. The Schnabel process has been reviewed by Harvey [9].
In Figure 2, we see the increase in dissolution rate between pure ammonia and AAC for one type of bottom ash.
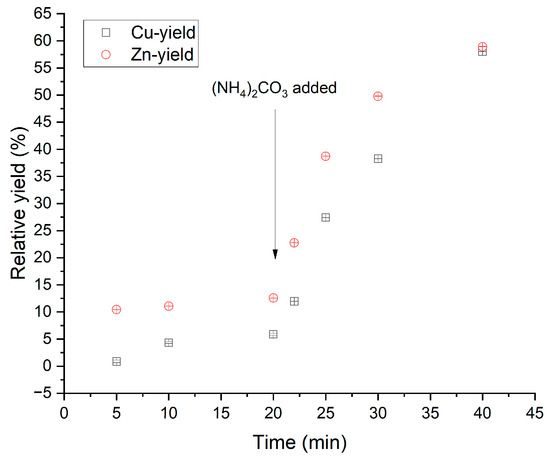
Figure 2.
Experiments performed as part of this work with pure ammonia and ammonia–ammonium carbonate. Ammonium carbonate was added when 20 min of the leaching time had passed. (Total NH3 = 4.68 M, T = 40 °C, pulp density = 100 g/L, stirring 250 rpm, NH3:CO3 = 3:1, V = 250.0 mL). Uncertainties are analytical standard deviations that do not represent the average of the sample contents.
We have the following reaction model for leaching with pure aqueous ammonia:
where M denotes Cu or Zn. But for AAC, we have:
Sum reaction: (3) + (4):
Employing the Law of Mass Action or the principle of Le Chatelier, we clearly see the advantage of AAC versus ammonia.
In addition, there are two simultaneous buffers: NH3/NH4+ and CO32−/HCO3−. Tests during leaching have shown quite stable pH levels at just below 9.8, just in the optimum pH region.
To recover the copper from the PLS, a very simple sedimentation process is possible. Being the most noble element in the PLS (an exception may be silver), adding elemental zinc to the solution will reduce Cu2+ to Cu0. This part is also a part of the Schnabel process, as reviewed by Harvey [9]. The dark blue color of the copper amine complex disappears, and finely divided copper is precipitated. Since initially there is zinc in the PLS, there will be an increase in the concentration. This process step is an option and has to be evaluated in competition with first the electrolysis of copper, then zinc and then the other, less noble metals present. Here, the purities of the metals will play a significant role in assessing the most economical and technical way to recover the metals. We have not yet performed this assessment.
It is also possible to force the precipitation of Zn(OH)2 by heating and evaporation of NH3. This recycles ammonia and recovers the zinc. According to Meng and Han [5], the pH interval needed to handle this precipitation is 7–8, which is quite narrow. We consider the zinc-reduction process to be more robust.
During the incineration of municipal waste, the temperature can rise to values sufficient to evaporate metals and metal oxides of Cd, Hg, Pb etc. In fly ash, there may, therefore, be more toxic heavy metals than in the volatile fraction left—the bottom ash. We also assessed other metals, e.g., Cd and Pb, but we focus on Cu and Zn in this work.
2. Materials and Methods
Since these kinds of raw materials are very variable in terms of content, it is impossible to provide exact and reliable yields. Therefore, we used XRF to determine the concentrations of the metals and accepted that the yields of copper and zinc will be uncertain. The yields were calculated from the volumes and concentrations of the PLS relative to the concentration of the solid samples as determined by XRF. However, for our samples, we have selected the procedures that are the most robust and provide the highest yields. The tested parameters were leaching duration, temperature, pulp density, ratio NH3/NH4, etc. Stirring was kept constant as long as the viscosity of the slurry was low enough to allow for good mixing. When this was no longer fulfilled, testing the parameter in question (pulp density) stopped.
2.1. Chemicals
Ammonia as a 28% aqueous solution NH3(aq) was purchased from VWR and used as such. Ammonium carbonate, (NH4)2CO3(s) as solid salt, was also purchased from VWR and used as such. Standards for chemical analyses were provided by Teknolab AS. For the dilution of samples and their preparation, 68% HNO3 purchased from VWR was used. The water used was Type II water (15.0 MΩcm) purified by Milli-Q® Integral Water Purification System from Merck Millipore (Burlington, MA, USA).
2.2. Instruments
The analytical instruments at our disposal were MP-AES, ICP-OES, XRF and XRD. Analyses of the aqueous samples were partly performed with Microwave Plasma Atomic Emission Spectroscopy (MP-AES) using Agilent 4100 MP-AES with Agilent SPS 4 autosampler (Mulgrave, Victoria, Australia) or with ICP-OES using a Horiba Jobin Yvon Ultima 2 instrument (Longjumeau, France). Analyses of solid samples by XRF measurements were performed by NOAH AS using a SPECTRO XEPOS 5 HE (Kleve, Germany), whereas XRD measurements were performed employing a Bruker D8 Discover with Bragg–Brentano geometry.
2.3. Solids, i.e., Fly and Bottom Ash
Samples of fly ash were all provided by NOAH AS, a Norwegian waste-handling enterprise (en.noah.no). The samples were all washed with water to recover dissolvable chlorides, i.e., NaCl, KCl and CaCl2. Thus, these ashes were depleted in chloride compared to ordinary fly ashes. The ashes were dried and used as such.
The bottom ashes were provided by four Norwegian waste handling facilities. They were sampled at a random time and are considered representative of the variation explained in the introduction. The ash particles are sintered, and to leach them, they were initially ground in a rotor mill to a particle size of <2 mm. Tests with both coarse and ground ash showed a clear advantage of grinding. The yields were 2–3 times higher when ground. The coarse material was leached slowly, but we did not know how long it would take before saturation. The ground ashes reacted faster and were thus better to work with.
3. Results and Discussion
To find the optimum ratio between NH3(aq) and (NH4)2CO3 on leaching fly ash, a series of tests where the ratio was altered was performed. The results for Cu and Zn are shown in Figure 3. As shown, both yield curves have a maximum and then decrease. The separation factor between Cu and Zn is increasing at the expense of yield. For Cu, the peak is close to:
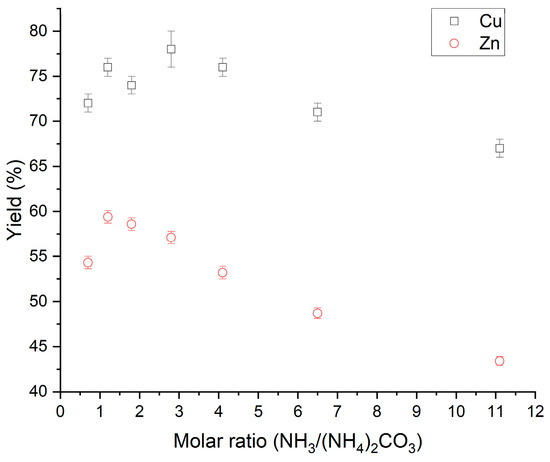
Figure 3.
Tests to find the optimum ratio between NH3(aq) and (NH4)2CO3 on leaching fly ash. (Temperature: 25 °C, pulp density 250 g/L, leaching time: 60 min).
The ratio between amine–ammonium and the total concentration of [NH3] + [NH4+] may play a role. In Figure 4, two different samples of bottom ash are shown for three different total concentrations. The ratio between ammonia and ammonium is 1.5 in all these tests. There is an increase in the yields for both Cu and Zn, going from 1.56 to 3.12 M, but the increase from 3.12 to 4.68 M is almost within uncertainty. However, the figure shows the significant differences that may exist between the bottom ashes from waste-handling incinerators.
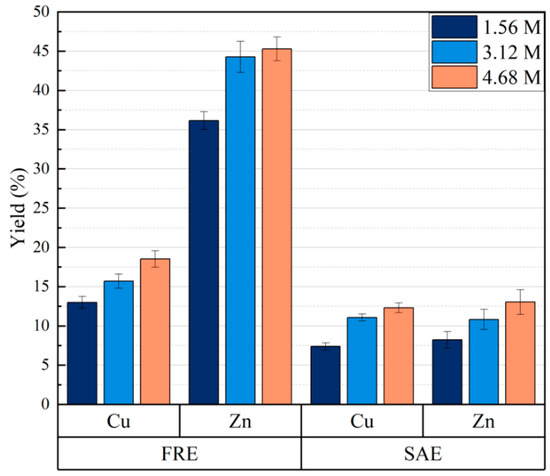
Figure 4.
Effect of total ammonia concentration (NH3 + NH4+) with regard to the yields of copper and zinc for two different types of bottom ash: FRE (left) and SAE (right). (Ambient temperature, pulp density = 100 g/L, leaching time: 40 min, stirring speed 250 rpm). The ratio between NH3 and NH4+ was 1.5 in all the tests).
Tests on the leaching of the bottom ash clearly showed that while the copper yields increased with increasing temperature, the yield of zinc decreased. We interpret this as when the activity of NH3(aq) increases and as the Cu-complex is stronger than the Zn-complex, there may be less available NH3 for the weaker complex. In addition, the competition from the OH- may increase, forming colloidal Zn(OH)2(s) and Zn(OH)42−.
4. Conclusions
We have clear indications that the combination of fly and bottom ash may represent potential sources for copper and zinc. There is a strong possibility that both materials can be leached by the same method and with the same parameters. The recommended lixiviant is ammonia–ammonium carbonate, AAC, where the molar ratio of ammonia to ammonium is 1.5, and the total ammonia + ammonium concentration is 1.6 M with a leaching time of 180 min.
Author Contributions
Conceptualization E.N.N. and D.Ø.E.; methodology. A.R.S. and E.N.N.; visualization: R.E.D., A.R.S. and E.N.N.; validation. R.E.D., A.R.S. and E.N.N.; investigation R.E.D., A.R.S. and E.N.N.; resources J.P.O. and D.Ø.E.; data curation R.E.D., A.R.S. and E.N.N.; writing—original draft preparation D.Ø.E.; writing—review and editing R.E.D., A.R.S. and E.N.N.; supervision D.Ø.E.; project administration J.P.O. and D.Ø.E.; funding acquisition J.P.O. and D.Ø.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received financial support from the Research Council of Norway-BIA through project No. 294543 (PRICE and the PRICE collaboration).
Institutional Review Board Statement
Not relevant.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting reported results have been included in the manuscript in the form of graphs.
Acknowledgments
The authors are grateful for the support from NOAH AS for the samples of fly ash and XRF analyses. We acknowledge the support from the four waste-handling enterprises for bottom ash samples, and from NOAH for the fly ash samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nedkvitne, E.N.; Eriksen, D.Ø.; Omtvedt, J.P. Grade and Tonnage Comparison of Anthropogenic Raw Materials and Ores for Cu, Zn, and Pb recovery. Resources 2023, 12, 33. [Google Scholar] [CrossRef]
- Nedkvitne, E.N.; Borgan, Ø.; Eriksen, D.Ø.; Rui, H. Variation in chemical composition of MSWI fly ash and dry scrubber residues. Waste Manag. 2021, 126, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Hjelmar, O.; van der Sloot, H.; van Zomeren, A. HP classification of European incinerator bottom ash (IBA). DHI & ECN Published by the Confederation of European Waste-to-Energy Plants (CEWEP) (February 2013).
- Choppin, G.R. Complexation of Metal Ions. In Solvent Extraction Principles and Practice, 2nd ed.; Rydberg, J., Cox, M., Musikas, C., Choppin, G.R., Eds.; Marcel Dekker Inc.: New York, NY, USA; Basel, Switzerland, 2004. [Google Scholar]
- Meng, X.; Han, K.N. The Principle and Applications of Ammonia Leaching of Metals—A Review. Miner. Process. Extr. Metall. Rev. 1996, 16, 38. [Google Scholar] [CrossRef]
- Liu, W.; Tang, M.; Tang, C.; He, J.; Yang, S.; Yang, J.; Chen, Y. Thermodynamic Research of Leaching Copper Oxide Materialswith Ammonia-ammonium Chloride-water Solution. Can. Metall. Q. 2010, 49, 131–145. [Google Scholar] [CrossRef]
- Bingöl, D.; Canbazoğlu, M.; Aydoğan, S. Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching. Hydrometallurgy 2005, 76, 55–62. [Google Scholar] [CrossRef]
- Rodriguez Rodriguez, N.; Gijsemans, L.; Bussé, J.; Roosen, J.; Önal, M.A.R.; Masaguer Torres, V.; Fernnandez, A.M.; Jones, P.T.; Binnemans, K. Selective Removal of Zinc from BOF Sludge by Leaching with Mixtures of Ammonia and Ammonium Carbonate. J. Sustain. Metall. 2020, 6, 680–690. [Google Scholar] [CrossRef]
- Harvey, T.G. The hydrometallurgical extraction of zinc by ammonium carbonate: A review of the schnabel process. Miner. Process. Extr. Metall. Rev. 2006, 27, 231–279. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).