1. Introduction
Microemulsions are thermodynamically stable isotropic dispersions of oil and water containing nanometer-sized droplets stabilized by surfactant(s). Microemulsions are promising carriers for targeted drug delivery. Since they contain an aqueous and oily phase, their advantage is the possibility of the simultaneous inclusion of both water- and oil-soluble biologically active substances. Unlike liposomes, microemulsions are formed spontaneously when the necessary components are mixed and, at a constant composition and temperature, they can exist indefinitely. The consequence of the thermodynamic stability of microemulsions is their advantages in terms of technology—these include simple methods of obtaining, the possibility of long periods of storage, and good reproducibility of properties. Most often, microemulsions are developed for oral administration and for application to the skin and mucous membranes [
1].
The nanostructures of lecithin, which is a well-known surfactant of natural origin and the main lipid component of cell membranes, can be used to develop drug carriers. The microemulsions of lecithin with various cosurfactants are considered as drug carriers. They have advantages such as biocompatibility, the ability to solubilize biologically active substances while maintaining their activity, and the ability to accelerate the transport of drugs through the skin. For example, for the transdermal delivery of tetracaine, direct and reverse microemulsions in the system lecithin–n-propanol–isopropyl myristate–aqueous solution of tetracaine were proposed; the type of microemulsion depended on the ratio of lecithin/propanol [
2,
3]
To obtain microemulsions of lecithin intended for medicine and cosmetics, vegetable oils and essential oils can be used. Vegetable oils with their own biological activity can be introduced into the composition of the organic phase of microemulsions. Among these oils, one can single out gac oil. Gac (
Momordica cochinchinensis) is a fruit plant grown in Southeast Asia. Gac fruit has a wide range of biological functions, including antioxidant, anti-cancer, anti-inflammatory, antimicrobial, and immunomodulatory properties. Thanks to such remarkable properties, gac fruits and their products attract the attention of scientists of various specialties [
4,
5,
6,
7].
The biological activity of gac oil can be complemented by the action of turmeric essential oil. The essential oil of turmeric, obtained from the rhizome of turmeric (
Curcuma longa), contains sequiterpenes such as sesquifellandrene, ar-curcumene, β-turmerone, and ar-turmerone; it also has an antioxidant effect [
8].
The aim of this work is to develop microemulsions in the soy lecithin–vaseline oil–gac oil–turmeric essential oil–water systems. Such microemulsions can serve as the basis for the creation of new medical and cosmetic products.
2. Materials and Methods
To obtain a microemulsion, soy lecithin “Moslecithin” with the phospholipid content of no less than 97 wt.% (Vitaprom, Moscow, Russia), oleic acid of “pure” grade (Khimmed, Moscow, Russia), medical Vaseline oil (Kazan Pharmaceutical Factory, Kazan, Russia), gac oil, and turmeric essential oil (Ha Noi Natural Essential Oil, JSC, Hanoi, Vietnam) were used.
To obtain a microemulsion, a weighed portion of lecithin was dissolved in a mixture of Vaseline oil and gac oil at a temperature of 50 °C and mechanically stirred for 1–1.5 h in a closed vessel. Then, the sample was cooled to room temperature, and oleic acid and turmeric oil were added. The required amount of water was added to the resulting oil solution. Solubilization of water was carried out at a temperature of 25 °C until completely solubilized. The homogeneity of the sample and the absence of water droplets and particles of the liquid crystal phase were controlled using an Axiostar plus polarizing optical microscope (Carl Zeiss AG, Oberkochen, Germany) at room temperature.
The hydrodynamic diameter of microemulsion droplets was determined via dynamic light scattering using a Zetasizer Nano ZS particle size analyzer (Malvern Instruments, Malvern, UK).
IR spectra were recorded on a Nicolet 380 IR-Fourier spectrometer (Thermo Scientific, Waltham, MA, USA). The measurements were made using the equipment of the Center for Collective Use named after D.I. Mendeleev.
Measurement of the dynamic viscosity of the samples was carried out using a rotational viscometer (rheometer) Haake Viscotester iQ, measuring device type “coaxial cylinders” CC25 DIN/Ti, with increasing shear rate. Before measurement, the sample was thermostated for 15 min.
The study of the kinetics of the release of water-soluble substances from the microemulsion was carried out via dialysis on the model of the water-soluble dye Rhodamine C. For dialysis, a regenerated cellulose tubular membrane M-Cel (Viscase, Pantin, France) with a pore size of 3.5 kDa was used.
3. Results and Discussion
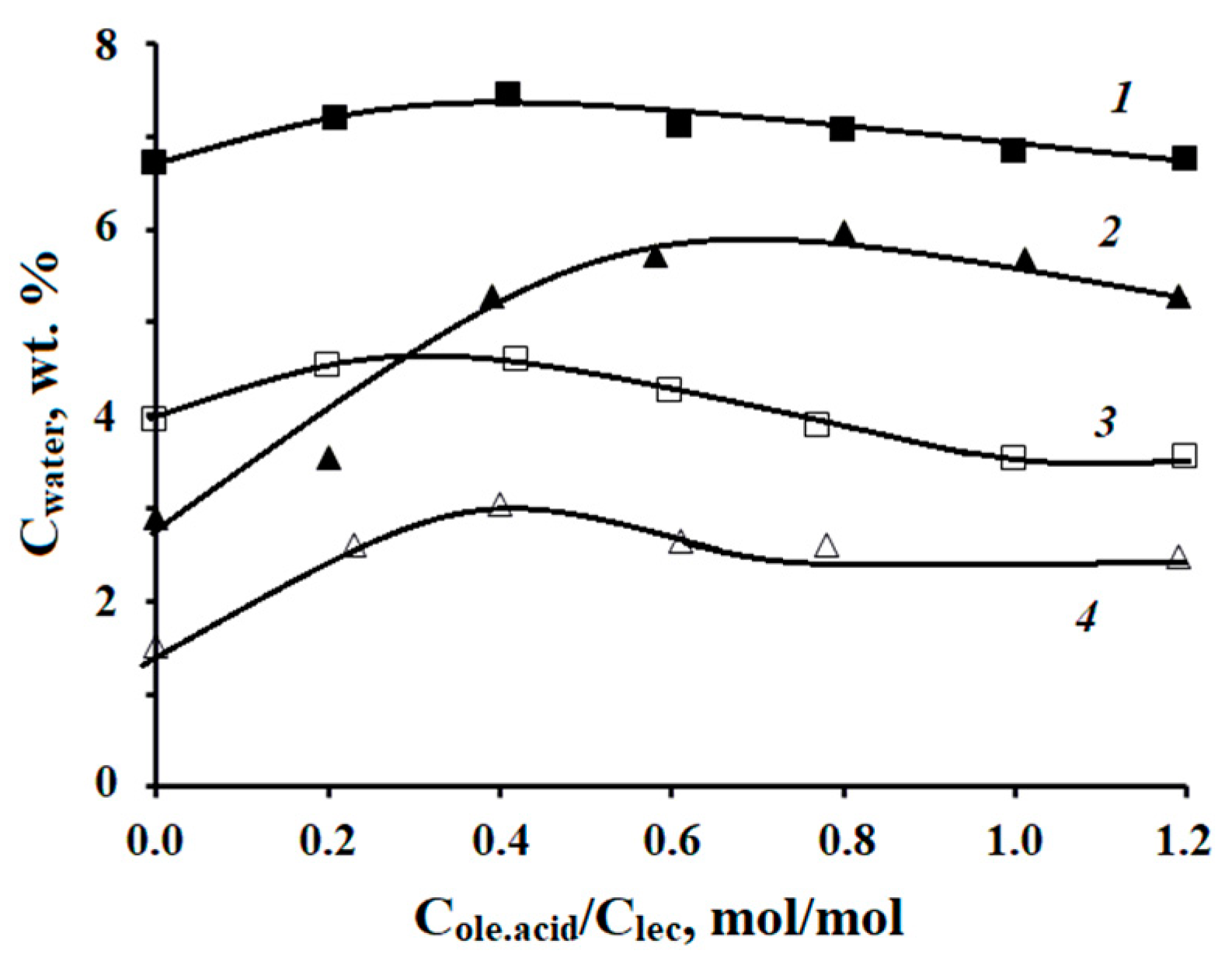
The water solubilization capacity of microemulsions in the lecithin–oleic acid–vaseline oil–vegetable oil–turmeric oil–water systems was studied. As vegetable oil, we used gac oil and avocado oil, which was proposed earlier in Ref. [
9]. The ratio of Vaseline oil/vegetable oil was 1:1 by weight, the concentration of turmeric essential oil in the organic phase was 4.5 wt.%, and T = 25 °C. The range from 0 to 1.2 of the ratios of the molar concentrations of oleic acid and lecithin was chosen based on the previously published data for microemulsions in the system lecithin–oleic acid–dodecane–water [
10]. In the entire range of C
ole.acid/C
lec ratios considered, at lecithin concentrations of 10 and 20 wt % in the organic phase, the microemulsion containing gac oils had a higher solubilization capacity with respect to water than the microemulsion with avocado oil, which was previously proposed in Ref. [
9]. At least 6.5 wt.% of water can be introduced into the lecithin microemulsion containing gac oil at a lecithin concentration of 20 wt.% in the organic phase, with the C
ole.acid/C
lec values ranging from 0.2 to 0.8; the maximum water content is observed at a ratio of C
ole.acid/C
lec equal to 0.4 (
Figure 1).
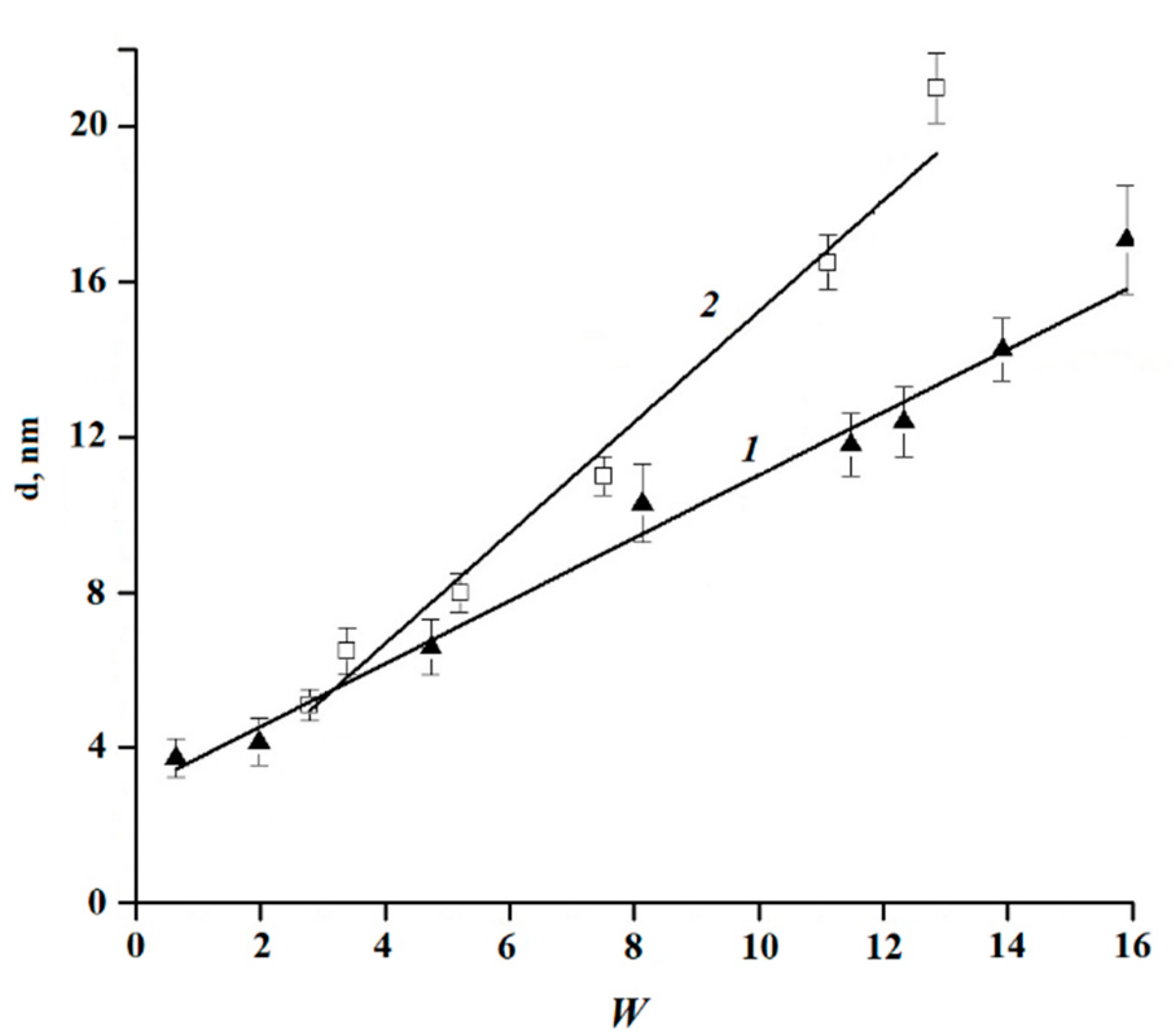
The droplet size of a few nanometers is typical for reverse microemulsions containing a small amount of water. To prove that the studied system is a thermodynamically stable microemulsion, the hydrodynamic diameter of the droplets of the sample was determined after heating to 60 °C and cooling to 25 °C, as well as after freezing at −20 °C and thawing (
Table 1). After heating to 60 °C and cooling, and after freezing at −20 °C and subsequent thawing, the structure of the microemulsion was restored, and the droplet size practically did not change.
The dependence of the hydrodynamic diameter of the droplets on the parameter W = C
water/C
lec was studied for the microemulsions containing various amounts of lecithin, oleic acid, and turmeric essential oil. Depending on the concentrations of water and lecithin, the hydrodynamic diameter of the microemulsion droplets varies in the range from 3 to 21 nm; a linear dependence of the diameter on W is shown in
Figure 2.
The IR spectra of the reverse microemulsions were obtained in the lecithin–oleic acid–Vaseline oil–gac oil–turmeric essential oil–water system, containing different amounts of water and having the same composition as the organic phase (wt.%) as follows: lecithin—20; oleic acid—4.5; vaseline oil—35.5; gak oil—35.5; essential oil of turmeric—4.5. The study was carried out at room temperature (~25 °C), with the W values equal to 4 and 14.
The spectra obtained are distinguished mainly by a wide band in the frequency range 3000–3700 cm
−1. Through an analogy with previous studies of reverse microemulsions [
11,
12], this band can be attributed to stretching vibrations ν(OH) associated with the existence of various types of water microemulsions in drops. The band of stretching vibrations ν(OH) in microemulsions has a wide asymmetric shape with centers at a frequency of 3376 ± 10 cm
−1 (at W = 14) and 3368 ± 10 cm
−1 (at W = 4). For a mixture of oils, this band is absent; for microemulsions, its intensity increases with the increasing water concentration.
Through an analogy with Refs. [
11,
12], the mole percentage of the water of each type was calculated as the ratio of the area of the Gaussian band corresponding to this type of water to the sum of the areas of all bands into which the band ν(OH) was decomposed. According to the calculation, for the microemulsion with W = 14, the proportion of bulk water was 36.5 mol %, the proportion of hydration water was 55.0 mol %, and the proportion of water in the hydrocarbon chains was 8.5 mol %. Thus, in the system under study, both bound (hydrated) and free (bulk) water are present in the droplets, which indicates its microemulsion nature and distinguishes it from reverse micelles.
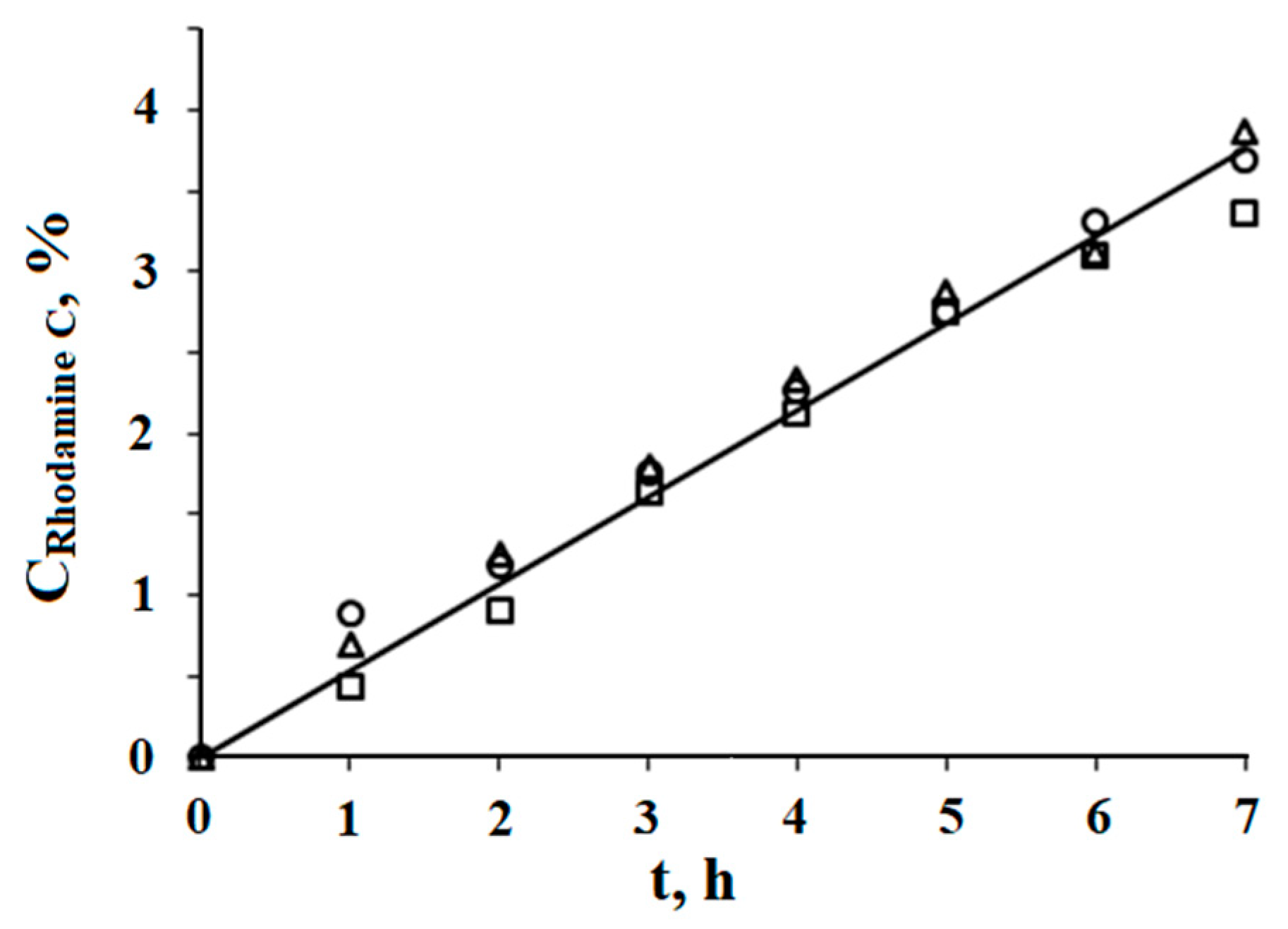
The release of water-soluble biologically active substances from the developed microemulsion containing 20 wt.% lecithin in the organic phase and 2.5 wt.% water (W = 4.74) was studied via dialysis. The study was carried out on the model of the water-soluble dye Rhodamine C; its concentration in the samples was 0.2 wt.%, the receiving medium was physiological saline, and three parallel experiments were carried out at T = 37 °C. The substance transfer rate was calculated using the formula V = m/(t∙S), where m is the mass of the released substance, t is the time interval, and S is the surface area through which dialysis takes place. The transfer rate of the water-soluble dye from the reverse microemulsion was 15.4 × 10
−3 g/(m
2∙h); approximately 3.2% of Rhodamine C was released in 6 h. These results are close to the data obtained earlier for the transfer of Rhodamine C from a similar reverse microemulsion in the lecithin–oleic acid–vaseline oil–avocado oil–tea tree oil–water system, where the rate was 14.3 × 10
−3 g/(m
2∙h) [
9] (
Figure 3).
The viscosity of this microemulsion slightly depended on the shear rate; in the shear rate range of 1.0–100 s−1 it was 0.072 Pa∙s at T = 25 °C and 0.043 Pa∙s at T = 37 °C.
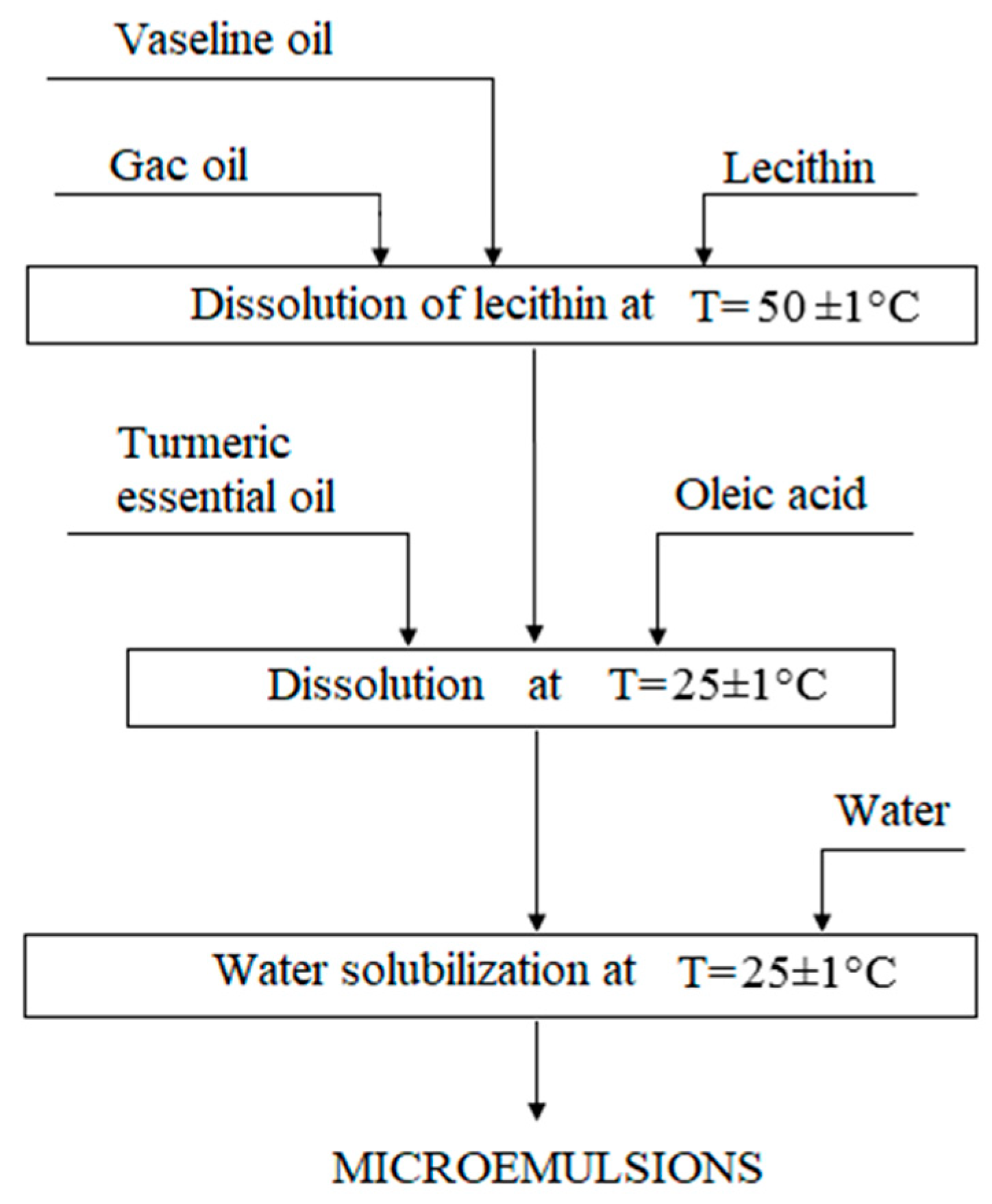
Based on the data obtained, a method was developed for obtaining a microemulsion of lecithin with gac oil and turmeric essential oil on a laboratory scale (one-time production of 100 g of the drug). To obtain microemulsions, it was proposed to dissolve lecithin in a mixture of vaseline and vegetable oils at a temperature of 50 °C, and then sequentially add essential oil and oleic acid, and then add water to the oil solution of lecithin at room temperature (
Figure 4). This technique makes it possible to introduce water-soluble biologically active substances into the composition of the microemulsion, which are unstable to heating, since the introduction of water or an aqueous solution occurs at room temperature.
4. Conclusions
It was shown that in order to obtain reverse microemulsions in the lecithin–oleic acid–vaseline oil–vegetable oil–essential oil–water systems, oil from the tropical gac plant (Momordica cochinchinensis) and turmeric (Curcuma longa) essential oil can be used. At least 6.5 wt.% of water can be introduced into the microemulsion at a lecithin concentration of 20 wt.% in the organic phase, the ratio of Vaseline oil and gac oil is 1:1 by weight, and the Cole.acid/Clec values are from 0.2 to 0.8; the maximum water content observed at Cole.acid/Clec is equal to 0.4. Depending on the concentration of water and lecithin, the hydrodynamic diameter of the microemulsion droplets varies in the range from 3 to 21 nm; a linear dependence of the diameter on W is shown. Both after heating to 60 °C and cooling, and after freezing at −20 °C and subsequent thawing, the structure of the microemulsion was restored, and the droplet size practically did not change. Using IR-Fourier spectroscopy, it was shown that for the microemulsion with the molar ratio of water and lecithin W=14, the fraction of bulk (free) water in the droplets was 36.5 mol %, the fraction of hydration water (bound to polar groups of surfactants) was 55.0 mol %, and the fraction of water trapped between hydrocarbon chains was 8.5 mol %. The resulting reverse microemulsions are characterized by a low rate of release of water-soluble substances. The rate of transfer of the water-soluble dye Rhodamine C from the microemulsion through the dialysis membrane into the physiological solution was 15.4 × 10−3 g/(m2∙h); in 6 h, approximately 3.2% of the dye was released.
A technique was developed for obtaining a microemulsion of lecithin with gac oil and turmeric essential oil on a laboratory scale (one-time production of 100 g of the composition). This technique makes it possible to introduce water-soluble biologically active substances that are unstable to heating into the microemulsion. Microemulsions containing biocompatible components, such as lecithin and oleic acid, as well as biologically active substances from gac oil and turmeric essential oil, can be used in medicine and cosmetics to develop drugs with anti-inflammatory and antioxidant effects, with a slow release of drugs.