Abstract
This paper proposes a model for the dependence of heat capacity of thin metal films on the temperature and on the number of atomic layers in these films directly. Model representations are based on the principles of statistical physics for solids and concepts of the distribution of principal quantum numbers in the system of oscillators distributed in solids at high temperatures, i.e., Bose–Einstein distribution. The calculations were performed based on the comparison of the Helmholtz free energy values for the various configurations of films and the number of layers in them. The main tool for the model implementation was the formation and further calculation of the partition function, being an expression of the distribution of principal quantum numbers in the complex system of a thin film. Calculations showed the existence of the optimal film thickness at which the maximum heat capacity was achieved. The calculations were performed based on a comparison of the values of the Helmholtz free energy for different film configurations and the number of layers in them. The main tool for implementing the model was the formation and further calculation of the partition function, which was an expression of the distribution of principal quantum numbers in the complex system of a thin film. The calculation results show the presence of a 15–20% increase in the heat capacity of thin films, corresponding to 400–600 atomic layers and the Dulong–Petit law, i.e., the comparison of exceeding heat capacity values with bulk objects for a certain temperature range. The heat capacity reaches the highest values in thin films of 30–50 atomic layers in thickness and exceeds the value of 3R by ~2.0 times.
1. Introduction
The development of microelectronic devices is currently associated with the creation of new materials of small size [1] in the order of nanometers. Microelectronic devices based on thin films are widely used in various industries [2], for example, in the manufacturing of printed circuit boards, microprocessors, laminated structures, and application of coatings to increase the corrosion- and wear-resistance of parts.
The performance capabilities of microelectronic systems depend on the thermal characteristics of thin film structures [1]. The physical properties of materials of such dimensions may vary several times relative to their bulk properties [3]. The accurate measurement of the thermophysical properties of individual thin films is important for the modeling and prediction of microsystem thermal characteristics [1].
The theoretical developments of thin film heat capacity dependence on the temperature and thickness at high temperatures are required to estimate the volume expansion coefficients from the Grüneisen ratios. When creating combined laminated structures with the use of thin metal films, this allows the synchronization of their expansion in accordance with temperature changes. Thus, any delaminations, deformations of structures and, as a result, their failure can be avoided.
Experimental studies show the higher values of the dependence of the thin film heat capacity compared to that of bulk samples [4,5]. Experimental data also show that, in addition to the temperature dependence, the heat capacity of thin films rises with the decrease in the film thickness [6,7], and its values exceed the heat capacity of bulk samples.
Along with the literature data for the experimental determination of the heat capacity of thin films [4,5,6,7,8,9,10], there are also the theories determining the heat capacity of thin films at low temperatures [11,12,13,14].
However, the theoretical determination of the dependence of thin film heat capacity on the temperature and thickness at high temperatures is still little studied. The paper [15] investigates the dependence of the Grüneisen parameter on film thickness and temperature via theoretical modeling and molecular dynamics simulations. This paper also explores the temperature dependence and dependence on thickness of the heat capacity of thin films, in order to calculate the Grüneisen parameter. However, calculations in [15] assume that the values of thin film heat capacity at the temperatures close to Debye temperatures are constant. This paper proposes an original model of the processes of formation of thin film heat capacity and description of the heat capacity dependence on the film thickness, i.e., on the number of atomic layers. The model in our study predicts a significant change in the heat capacity of thin films, depending on the thickness at temperatures close to the Debye temperature. Our model also assumes a change in the temperature dependence of the thin film heat capacity of the Dulong–Petit law. These circumstances agree with the experimental data [4,5,6,7].
2. Modeling of Heat Capacity of Thin Films
The laminated structure of thin films is described using the formalism of oscillating structures, i.e., the atomic layers of films poorly interacting with each other. The distribution of energies over the corresponding oscillators in this structure obeys the Bose–Einstein distribution [16]. We also assume that the Helmholtz free energy can be described in terms of partition functions used in the Boltzmann distribution [16].
According to the known concepts [16], the free energy of a system of oscillating structures (in this case, atomic layers in crystal objects) can be represented as
where T is temperature; ℏ is Planck’s constant; a is the summation over the layers of the crystal system; ωa is the typical oscillation frequency in layer a; and summation over s reflects the energy distribution over oscillators distributed in layer a corresponding to the distribution of principal quantum numbers. Since this distribution at high temperatures obeys the Bose–Einstein distribution, summation is carried out between 1 and ∞. Furthermore, when we use the technique applied in the derivation of the Einstein’s formula [17]:
The known expression for the crystal structures of solids is obtained as shown below [16]:
Here, we are referring to high temperatures T > 273 K, i.e., the relationship T ≫ ℏωa is satisfied, and the exponent is expanded in a series up to the first term:
Further, using the expression for the geometric mean in the logarithmic representation :
where ϖ is the geometric mean frequency and n is number of layers over which the averaging is performed.
Then, in accordance with the Debye temperature concepts, Θ = ℏωD, where ωD is the Debye frequency. It should be emphasized that the Debye temperature reflects the thermal properties inherent in specific crystal structures of solids. Therefore, considering the geometric mean frequency as :
Next, when we use the Landau formalism, the expression for free energy is formed based on other considerations. To achieve this, we write the expression for the Boltzmann free energy of a certain system comprising n oscillators and n oscillating layers, expressed in terms of the partition function:
The division by n! is introduced in (7), proceeding from the assumption of indistinguishability of oscillators in the oscillating system as a whole. Further, Equation (7) can be written as:
where the sum is replaced by the integral over the phase space τ of coordinates and momenta q an p, with e as the base of the natural logarithm.
Selecting, in (8), the coordinate component in the phase space [16]:
where V is volume of the system and f(T) is a certain function depending on the temperature. Using the relationship , , where E is energy and S is entropy, we obtain the expression for the energy and heat capacity:
Equation (10) allows the determination of the function f(T) per one oscillating structure, by solving the following equation:
After the successive integration of the second-degree differential Equation (11), we obtain:
where η is the certain integration constant, which definitely depends on the number of oscillators in the system, since the function f(T) as a whole depends on this number. Therefore, η is an expression of this dependence on the number of oscillators. In this approximation, we present it in the simple form of η∼αn. ε0 is the certain additive initial energy which does not depend on the temperature. Furthermore, substituting Equation (12) into Equation (9) and equating both expressions for the free energy Equation (6) and Equation (9), we obtain:
After transformation of Equation (13), we obtain:
From Equation (14), we obtain an expression determining the heat capacity of the film depending on the number of layers n:
The integration constants and α are formed by varying from considerations of tendency of CV to the value of 3R, i.e., to the fulfilment of the Dulong–Petit law at n→∞.
Moreover, under the sign of the logarithm in Equation (14), there is the product eV, with V as the volume under the unit surface of one film layer. If we evaluate the linear size of the side as interatomic distance in the crystal lattice of 10−9 m, then the corresponding value is V~10−27 m3 and a is the constant of the crystal lattice. Taking into account the above estimates of the value , where , Equation (15) can be rewritten as:
where , b(T) is the correction factor reflecting the temperature dependence of the lattice cell expansion, according to our estimates, ~1.2 per each 100 K. The requirement applied to the constant C allows us to estimate the parameter α~0.2.
3. Results and Discussion
Figure 1 shows the dependence of CV~f(n) for the real values θ~400 K and T~350 K, 450 K, 550 K, 650 K. The graphical interpretation of results of the studies performed with the use of the proposed model representations indicates the fairly good interpolation and generalization of the disparate experimental data [4,5,6,7,8,9,10].
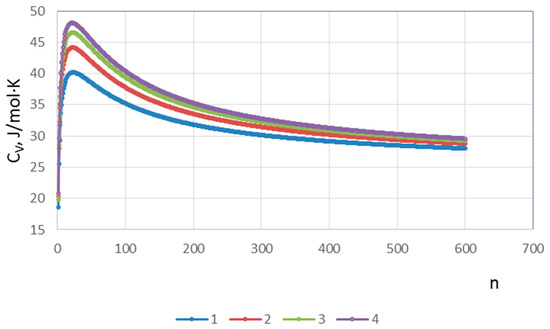
Figure 1.
Dependence of thin film heat capacity on the number of atomic layers of the film and temperature: 1—350 K; 2—450 K; 3—550 K; and 4—650 K.
In fact, a number of assumptions used the construction of a model to provide an opportunity to adequately explain the origin of the excess heat capacity of thin films and to trace the dynamics of changes in the heat capacity depending on the film thickness and temperature. Figure 1 shows a sharp increase in the heat capacity values with the decrease in the number of atomic layers in the film and, as a consequence, the excess of 3R values, i.e., the Dulong–Petit ratio. Furthermore, when the number of layers increases, the heat capacity values approach the values characteristic of bulk structures. The temperature dependence of excess heat capacity is most pronounced in thin films of 30–50 atomic layers in thickness and exceeds the bulk sample heat capacity by ~2 times. The excess heat capacity also occurs in thin films of 400–600 layers thick, exceeding the bulk sample heat capacity by a factor of 1.15–1.20. These aspects agree well with the experimental data [4,5,6,7].
Thus, the fairly traditional description of the properties of solids based on the representation of free energy in terms of partition functions, the quantum-mechanical distribution of the oscillator energy, and the corresponding values of the average principal quantum numbers, allow us to proceed to the characteristic thoroughly studied, i.e., the Debye temperature. This circumstance provides an opportunity to significantly expand the application of the model in terms of predicting the physical properties of the thin films of a wide range of metallic structures.
The proposed model representations allow the further prediction of the temperature dependence of the heat capacity and the dependence of the volume expansion coefficient in connection with the Grüneisen law. The degree of approximation of the proposed model can be increased using the specific values of the volume (10−27 m3) and the crystal lattice constant (10−9 m) in the calculations, instead of their generalized parameters, and considering the structure of the crystal lattice for a particular material.
4. Conclusions
The paper presents a model describing the dependences of thin film heat capacity on the temperature and thickness at high temperatures. The presented model uses a limited set of parameters, allowing the performance of the calculations with sufficient quality. The main parameters are the Debye temperature and the crystal lattice cell volume. These parameters integrally characterize the object of research, greatly expanding the prediction capabilities of the presented model.
According to the model concept, there is a significant deviation from the Dulong–Petit law observed in thin films at high temperatures. The heat capacity reaches the highest values for thin films that are ~40 atomic layers thick and exceeds the value of 3R by a factor of ~2.0.
Thin films of ~600 atomic layers demonstrate the excess of 3R values by a factor of ~1.15–1.20. With the increase in thickness of thin films, heat capacity values approach those of bulk samples.
The temperature dependence of thin film heat capacity close to the quadratic capacity is observed.
The original approach proposed in this paper is to study the heat capacity dependence directly on the number of layers, instead of the film thickness. It provides an opportunity to examine the values of heat capacity and temperature properties of very thin films (5–10 atomic layers).
Author Contributions
Conceptualization, V.S. and Y.S.; methodology, V.S. and Y.S.; software, V.S.; validation, V.S., and Y.S.; formal analysis, V.S. and Y.S.; investigation, V.S.; resources, V.S.; data curation, V.S.; writing—original draft preparation, V.S. and Y.S.; writing—review and editing, V.S. and Y.S.; visualization, V.S.; supervision, V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lugo, J.M.; Oliva, A.I.; Riveros, H.G.; Ceh, O. Heat capacity determination of metallic thin films using temperature profiles at room conditions: Theory. In Proceedings of the 2010 7th International Conference on Electrical Engineering Computing Science and Automatic Control, Tuxtla Gutierrez, Mexico, 8–10 September 2010; pp. 504–509. [Google Scholar] [CrossRef]
- Jain, A.; Goodson, K.E. Measurement of the Thermal Conductivity and Heat Capacity of Freestanding Shape Memory Thin Films Using the 3ω Method. J. Heat Transfer. 2008, 130, 102402. [Google Scholar] [CrossRef]
- Cahill, D.G.; Ford, W.K.; Goodson, K.E.; Mahan, G.D.; Majumdar, A.; Maris, H.J.; Merlin, R.; Phillpot, S.R. Nanoscale Thermal Transport. J. Appl. Phys. 2003, 93, 793–818. [Google Scholar] [CrossRef]
- Yu, J.; Tang, Z.; Zhang, F.; Ding, H.; Huang, Z. Measurement of the Heat Capacity of Copper Thin Films Using a Micropulse Calorimeter. J. Heat Transfer. 2010, 132, 012403. [Google Scholar] [CrossRef]
- Lugo, J.M.; Rejón, V.; Oliva, A.I. Specific Heat Determination of Metallic Thin Films at Room Conditions. J. Heat Transfer. 2015, 137, 051601. [Google Scholar] [CrossRef]
- Yu, J.; Tang, Z.; Zhang, F.; Philip, C.C.H.; Wang, L. Measurement of heat capacity of al thin film using a micro bridge calorimeter. Zhongguo Jixie Gongcheng/China Mech. Eng. 2005, 16, 168–170. [Google Scholar]
- Li, Q.; Narasaki, M.; Takahashi, K.; Ikuta, T.; Nishiyama, T.; Zhang, X. Temperature-dependent specific heat of suspended platinum nanofilms at 80–380 K. Chin. Phys. B 2016, 25, 114401. [Google Scholar] [CrossRef]
- Jun, Y.; Zhen-An, T.; Feng-Tian, Z.; Guang-Fen, W.; Li-Ding, W. Investigation of a Microcalorimeter for Thin-Film Heat Capacity Measurement. Chinese Phys. Lett. 2005, 22, 2429–2433. [Google Scholar] [CrossRef]
- Zhang, M.; Efremov, M.Y.; Olson, E.A.; Zhang, Z.S.; Allen, L.H. Real-time heat capacity measurement during thin-film deposition by scanning nanocalorimetry. Appl. Phys. Lett. 2002, 81, 3801–3803. [Google Scholar] [CrossRef]
- Queen, D.R.; Hellman, F. Thin film nanocalorimeter for heat capacity measurements of 30 nm films. Rev. Sci. Instrum. 2009, 80, 063901. [Google Scholar] [CrossRef] [PubMed]
- Lifschitz, I.M. On the heat capacity of thin films and needles at low temperatures. J. Exp. Theor. Phys. 1952, 22, 471–474. [Google Scholar]
- Gospodarev, I.A.; Syrkin, Y.S. Low-temperature heat capacity of thin films. Low Temp. Phys. 1983, 9, 989–993. [Google Scholar]
- Huang, M.; Chang, T.; Liu, C.; Yu, C. A theoretical study of the specific heat and Debye temperature of low-dimensional materials. Int. J. Heat Mass Transf. 2008, 51, 4470–4479. [Google Scholar] [CrossRef]
- Tosić, B.S.; Setrajcić, J.P.; Mirjanić, D.L.; Bundalo, Z.V. Low-temperature properties of thin films. Phys. A Stat. Mech. Appl. 1992, 184, 354–366. [Google Scholar] [CrossRef]
- Jiang, Y.C.; Sun, S.; Zhang, T.Y. Thickness- and temperature-dependent Grüneisen parameter in thin films. Nanoscale 2021, 13, 9853–9863. [Google Scholar] [CrossRef] [PubMed]
- Landau, L.D.; Lifshits, E.M. Statistical Physics; Pergamon Press: London, UK, 1958. [Google Scholar]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; Wiley: New York, NY, USA, 2005. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).