1. Introduction
Colloidal quantum dots (CQDs) are nanocrystal semiconductors whose surface is covered with organic compound shell ligands. Due to the quantum dimensional effect, their optical properties depend on the diameter of the semiconductor core.
Colloidal quantum dots of mercury chalcogenides make solution-based materials which are of interest because of their mid-IR spectral range. They may have potential for making optoelectronic devices due to their low price and simple method of production [
1].
Colloidal quantum dots (CQDs) of mercury telluride have attracted a lot of attention in the last decade because of their unique properties [
2]. Mercury telluride-based CQDs are promising candidates for use in various fields of engineering and science due to their incomparable combination of a large radius of the Boron exciton (30 nm) and the band gap (0 eV) for bulk material. This ensures the spectral rearrangement of the properties of the CQDs from the near to far IR range. On the basis of this material photodetectors, lasers and applications in telecommunication devices are being actively developed [
3,
4,
5].
Mercury sulfide CQDs are a material that has been less investigated, and based on our research, interesting results have been obtained. In comparison to mercury telluride, which is semimetal in bulk form, mercury sulfide bulk material presents a gap, and there are two crystalline forms of it (zinc blend and cinnabar) which could have chiral optical characteristics [
1,
2].
Photosensitive thin films are created from solutions of colloid quantum dots of mercury chalcogenide for use in photo devices. The composition of the ligand shell strongly affects the photoelectric properties of thin films [
6].
The purpose of this research to fabricate photoresistors based on mercury sulfide and mercury telluride colloidal quantum dot solutions, and to define their properties. Using atomic force microscopy (AFM) and volt-ampere characteristics (VAC) measurements, the two materials (HgTe and HgS) could be analyzed.
2. Materials and Methods
A series of experiments with application of layers of CQDs based on HgTe and HgS on glass substrates and electrodes, followed by exchange of ligands, were performed.
The application of a solution of colloidal quantum dots is carried out using dip-coating and spin-coating (35 s, 2500 rotations/min) methods. After each application of the CQDs layer, the original oleate ligand shell was replaced with the following ligands: S2−, SCN−, I−, ethanditiol-1,2.
The procedure for changing ligands to ethanditiol-1,2 (EDT) was performed according to the following scheme.
The sample was placed for 30 s in a solution of EDT/HCl/isopropanol (concentration 1/1/100 in volume);
The sample was placed for 30 s in pure isopropanol to wash away the ligand residues from the previous stage.
Procedures for changing ligands to S2−, I− and SCN− were carried out using an analogical method with proper solutions.
When layers of CQDs of HgTe and HgS were prepared on glass substrate, the reliefs of the surfaces of thin films were analyzed using an atomic force microscopy (AFM) method. Using AFM, the uniformity of the application of layers, the roughness of surface and the thickness of the films were determined. After determining the thickness of the layers of the samples with AFM, the procedures of application and exchange of various ligands were carried out on electrodes. Photosensitive films were created by applying layers of colloidal quantum dots of mercury chalcogenides to golden electrodes. As part of the work, measurements of both dark volt–ampere characteristics (VAC) and light VAC were made, when illuminated with a laser at 980 nm, for thin films of mercury chalcogenides. After replacing the original shells with I−, S2−, SCN− and ethandithiol-1,2, the obtained results were analyzed.
3. Results and Discussion
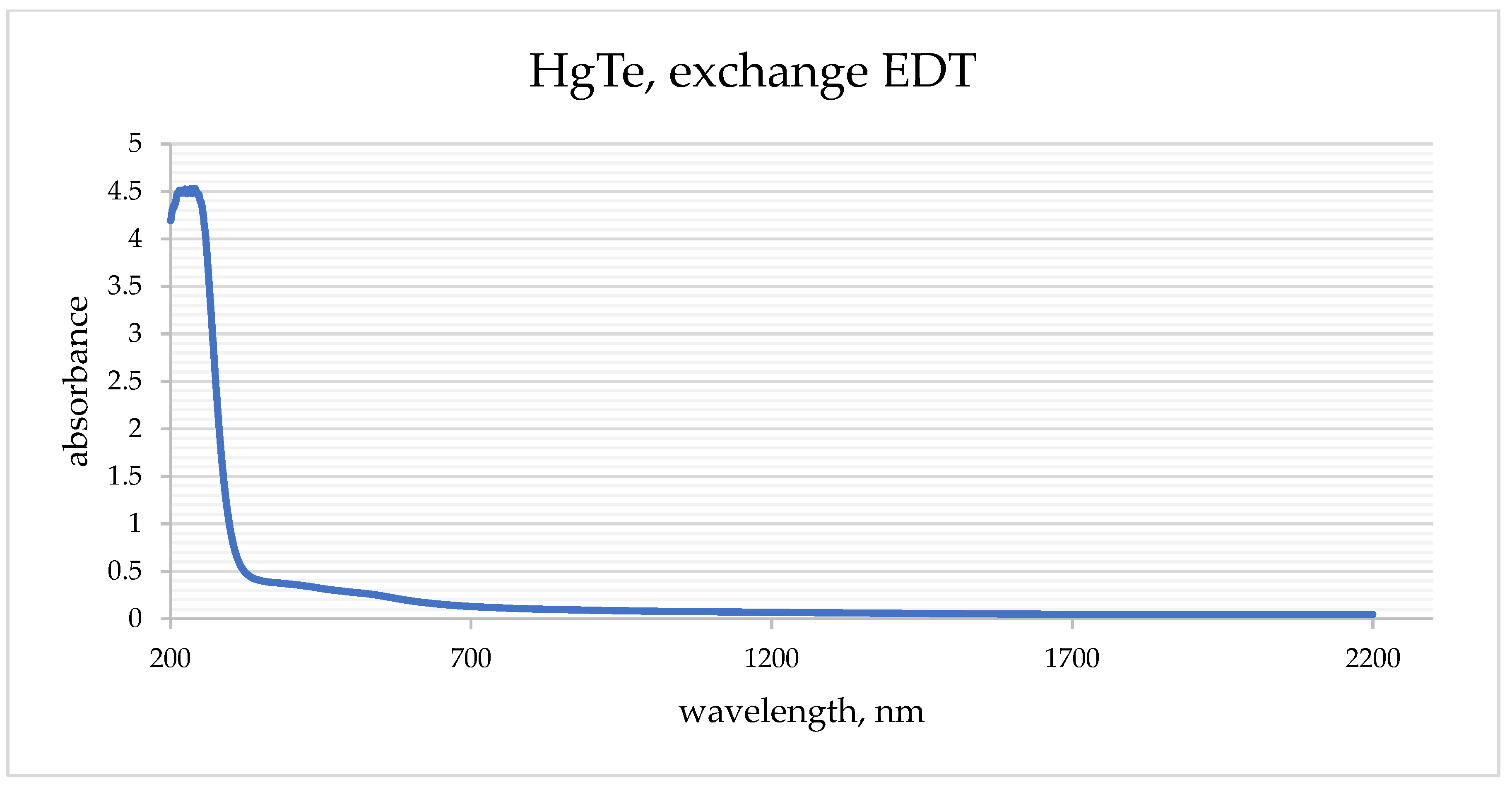
Thin films were prepared based on solutions of colloidal quantum dots using four different ligand exchanges. The optical density of prepared films was measured (
Figure 1).
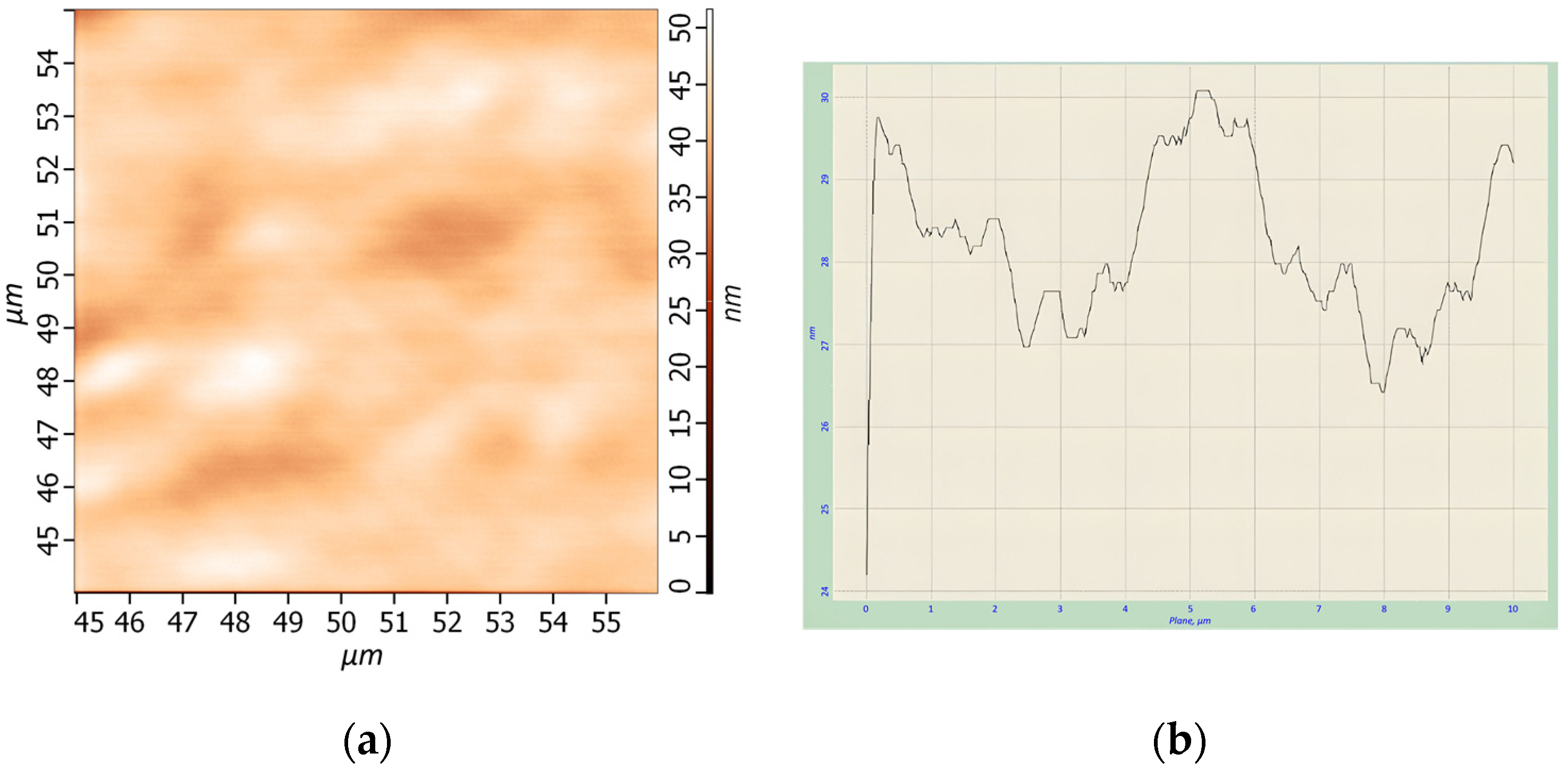
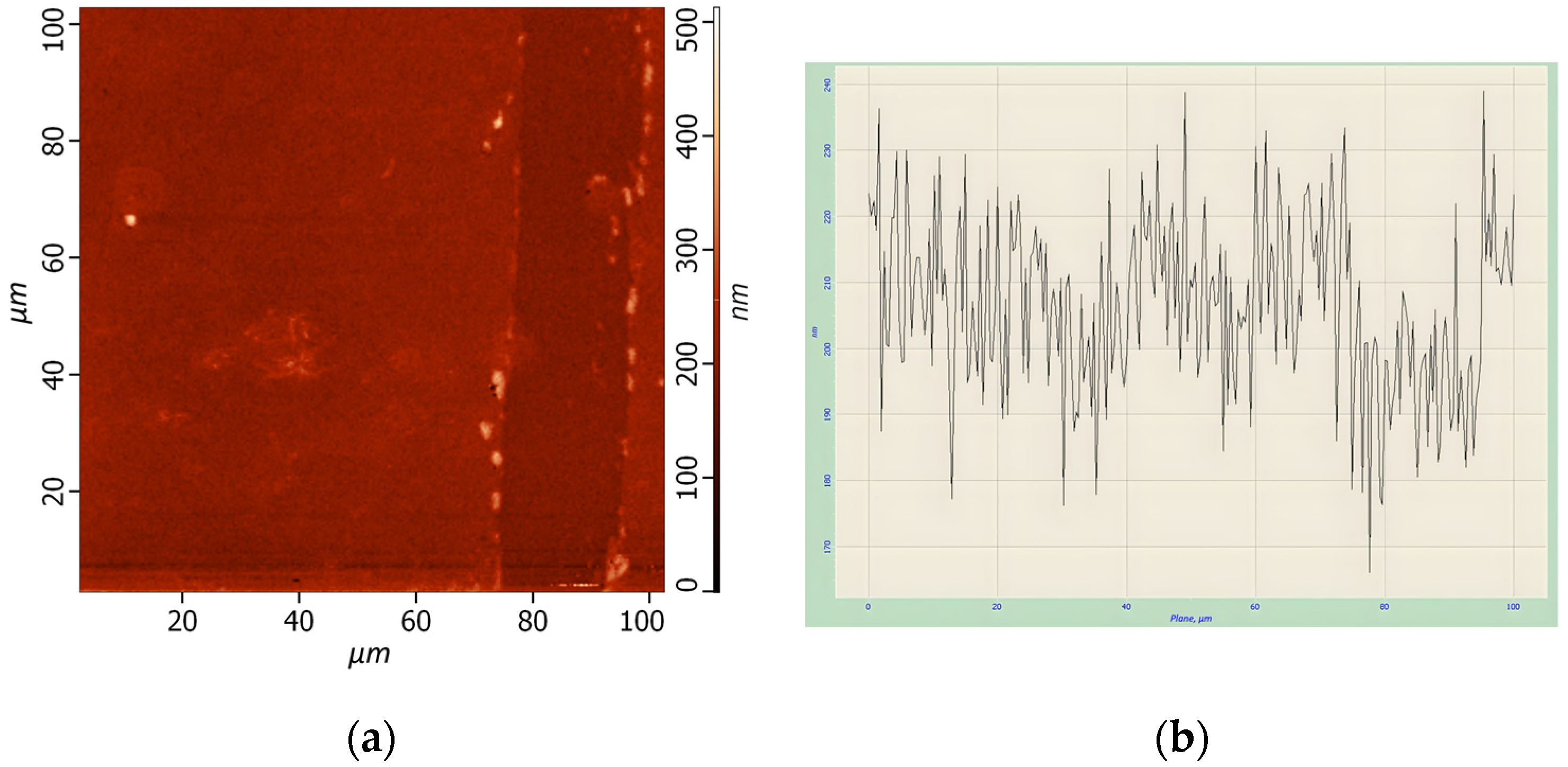
The arranged thin films were analyzed using atomic force spectroscopy (AFM). AFM analysis of one of samples of HgTe CQDs is presented in
Figure 2.
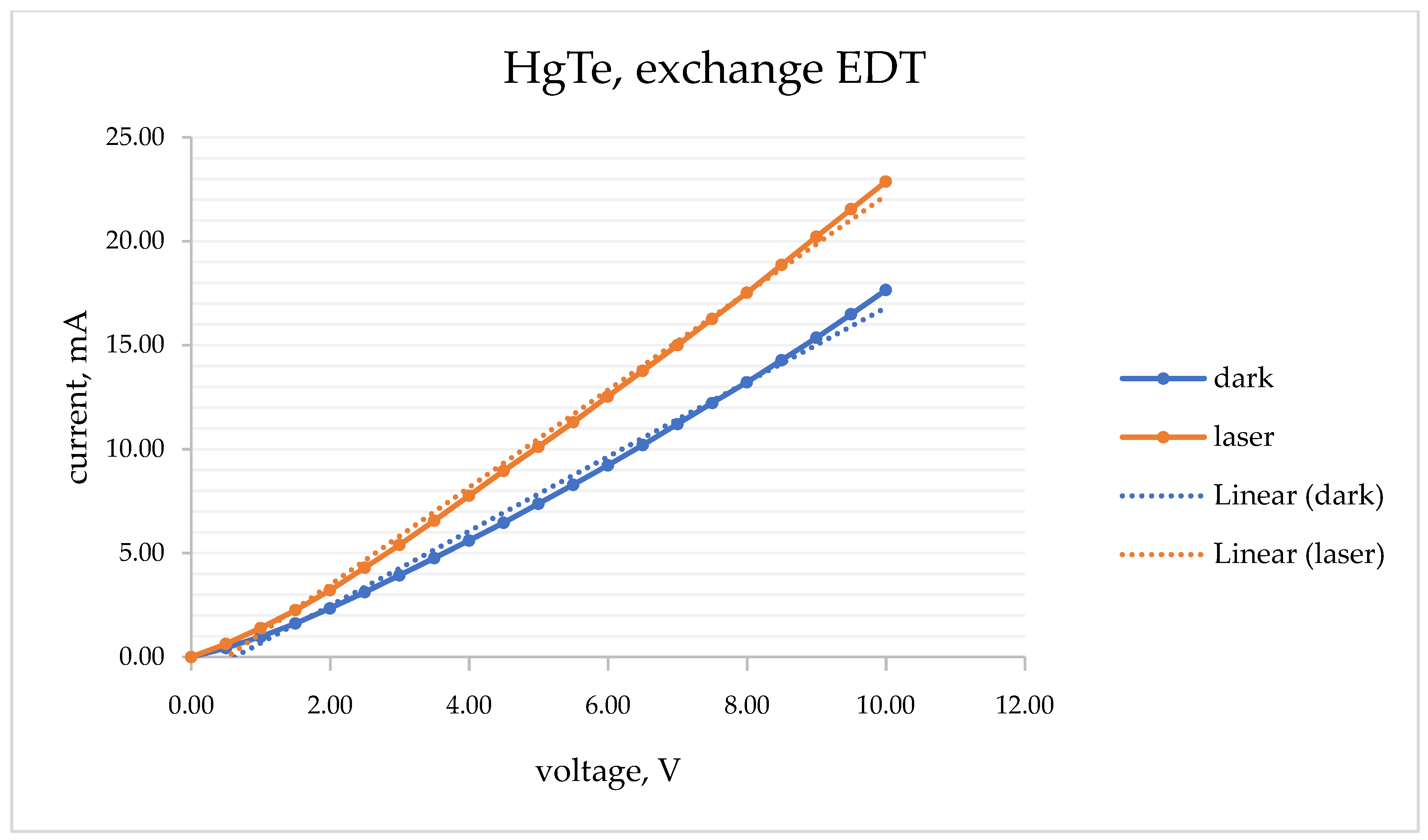
Based on the measurements of VAC, graphs of the current–voltage dependence for each electrode and different ligand substitution in both darkness and laser lighting conditions were constructed. In the case of HgTe, experiments were performed on each electrode in November 2021 and March 2022 to examine how the properties of photoresistors change with time. A photoresistor with ethanditiol-1,2 ligand exchange saved its properties in both experiments. In the cases of SCN
− and S
2− ligand exchange, the photoresistors’ resistance in the dark is less than with laser lightning (November experiment), and they lost their photosensitive properties by March. The iodide-substituted photoresistor did not have photosensitivity, and its resistance did not change over time. In the case of different substitutions, the current–voltage dependence functions have different forms. A classical photosensitive element (photoresistor) with linear CV function was obtained in the case of EDT ligand exchange (
Figure 3). The results of AFM and VAC measurements are shown in
Table 1.
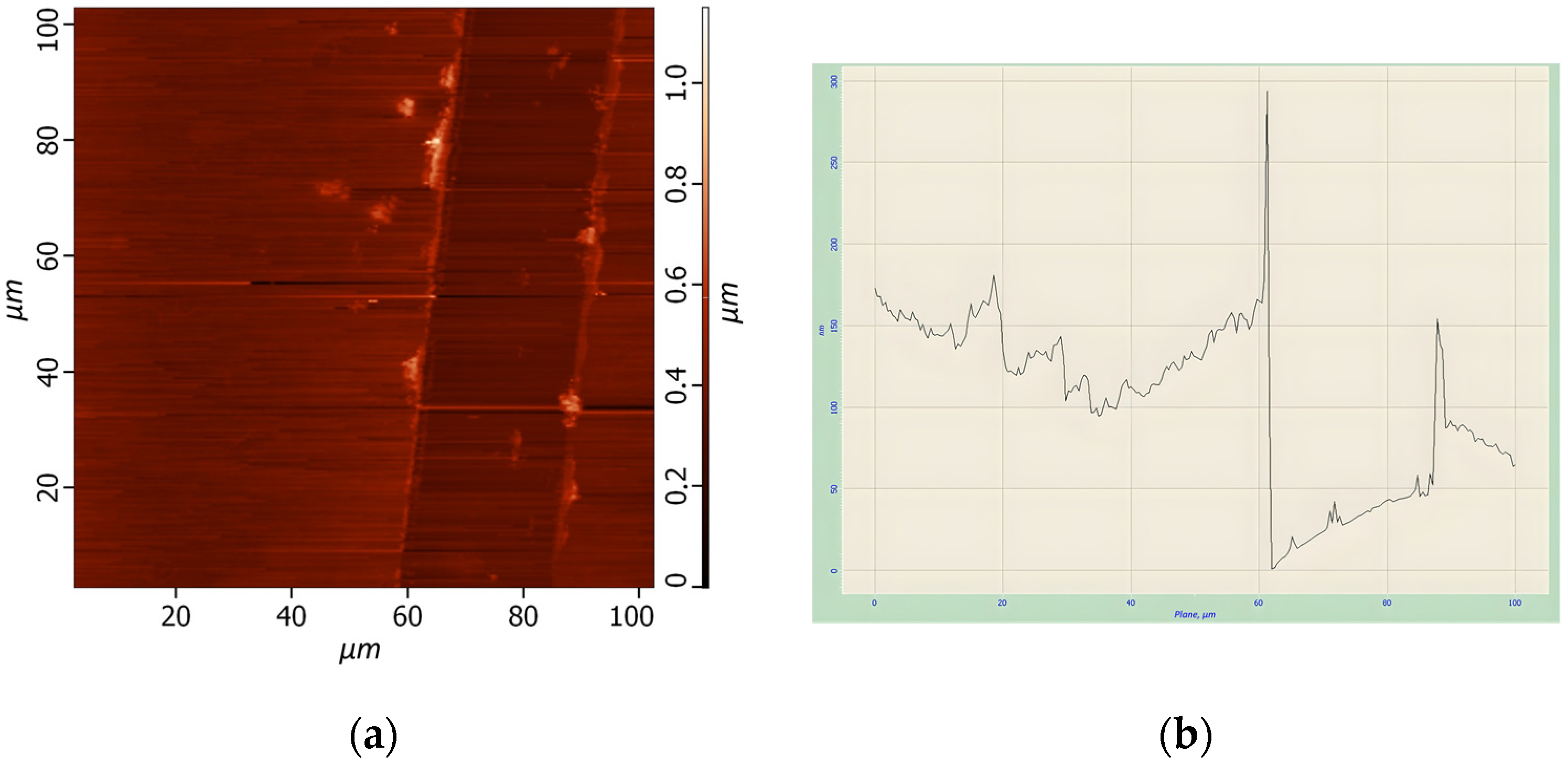
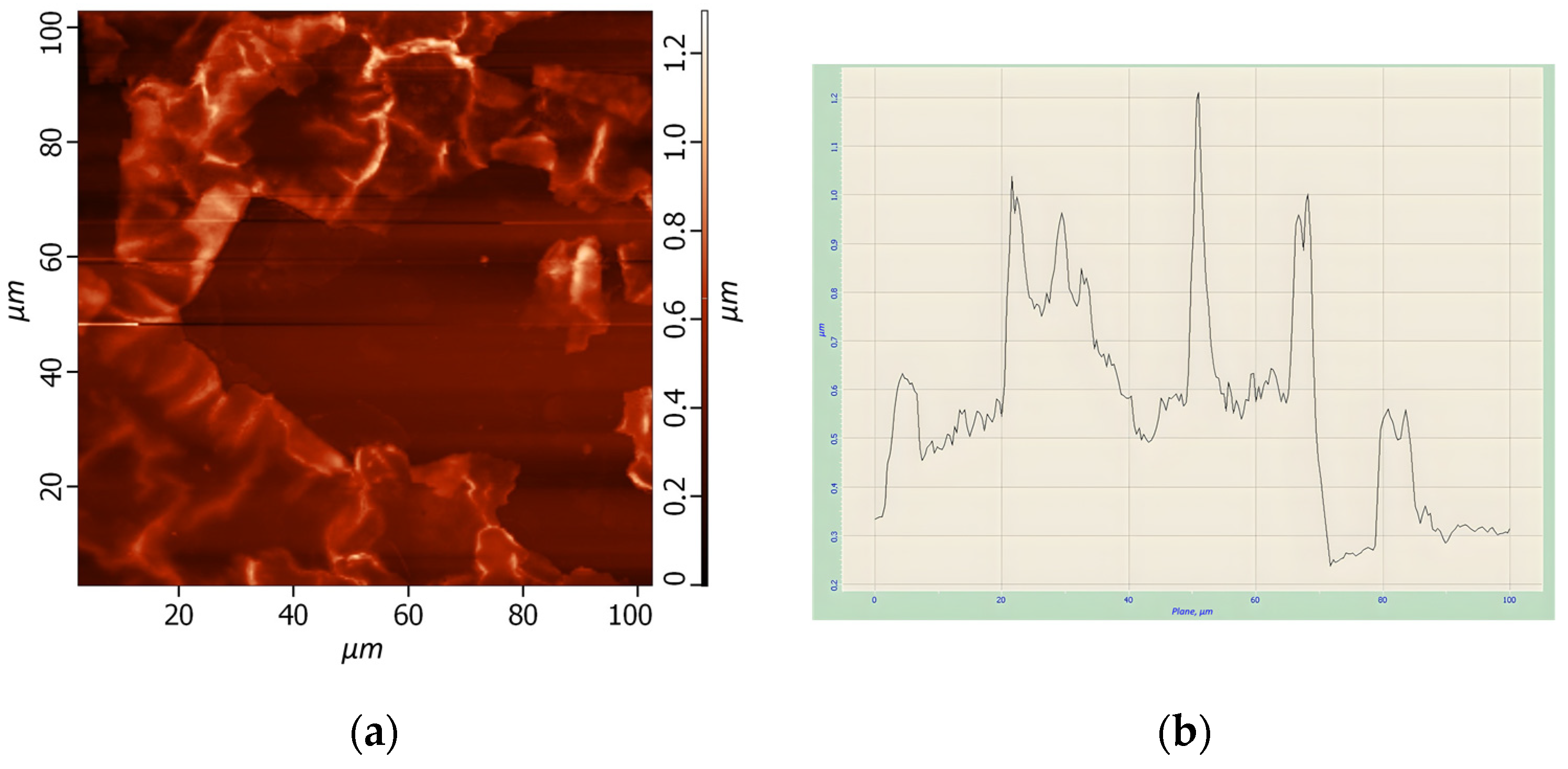
The solutions of quantum dots of HgS were synthetized using different parameters. By combining those parameters, several different samples were used for preparing thin films, and the results of AFM analysis of them are presented in
Table 2. AFM images and analysis of several samples were demonstrated on
Figure 4,
Figure 5 and
Figure 6.
4. Conclusions
In our experiments, we succeeded in producing layers of mercury chalcogenides colloidal quantum dots on glass substrates using spin-coating and dip-coating. The thin film deposition using spin-coating was optimized. The impact of the synthetic procedure of quantum dots, solvent and concentration of colloidal solution on the thin films’ properties was analyzed. It was found that concentrated solutions of mercury chalcogenides in tetrachloroethylene are best suited to the preparation of homogenous thin films with a roughness below 10 nm. The proper solutions of HgTe and HgS were determined using AFM, and utilized in the preparation of photoresistors by applying layers of CQDs with various ligand exchanges on electrodes. The photoelectrical properties of photoresistors were determined, based on HgTe CQDs in the case of EDT ligand exchange, using VAC measurements.
Author Contributions
Conceptualization, T.M., I.A.S. and V.S.P.; methodology, T.M.; software, T.M.; validation, T.M., A.A.M. and I.A.S.; formal analysis, T.M.; investigation, T.M. and A.A.M.; resources, T.M.; data curation, T.M.; writing—original draft preparation, T.M.; writing—review and editing, I.A.S.; visualization, T.M.; supervision, I.A.S.; project administration, V.S.P.; funding acquisition, V.S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was performed with the support of Ministry of Science and Higher Education of the Russian Federation, under the agreement no. 075-03-2022-207/10 dated 11 March 2022 (project No. FSMG-2022-0034).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, M.; Guyot-Sionnest, P. Reversible Electrochemistry of Mercury Chalcogenide Colloidal Quantum Dot Films. ACS Nano 2017, 11, 4165–4173. [Google Scholar] [CrossRef]
- Gréboval, C.; Chu, A.; Goubet, N.; Livache, C.; Ithurria, S.; Lhuillier, E. Mercury Chalcogenide Quantum Dots: Material Perspective for Device Integration. Chem. Rev. 2021, 121, 3627–3700. [Google Scholar] [CrossRef] [PubMed]
- Keuleyan, S.; Guyot-Sionnest, P.; Delerue, C.; Guy, A. Mercury Telluride Colloidal Quantum Dots: Electronic structure, size-dependant spectra and photocurrent detection up to 12 µm. ASC Nano 2014, 8, 8676–8682. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Haipeng, L.; Abdelazim, N.; Zhu, Y. Mercury Telluride Quantum Dot Based Phototransistor Enabling High Sensitivity Room Temperture Photodetection at 2000 nm. ASC Nano 2017, 11, 5614–5622. [Google Scholar] [CrossRef] [PubMed]
- Shuklov, I.A.; Razumov, V.F. Lead chalcogenide quantum dots for photoelectric devices. Russ. Chem. Rev. 2020, 89, 379–391. [Google Scholar] [CrossRef]
- Boles, M.A.; Ling, D.; Hyeon, T.; Talapin, D.V. The surface science of nanocrystals. Nature Mater. 2016, 15, 141–153. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).