Natural Clinoptilolite Nanoplatelets Production by a Friction-Based Technology †
Abstract
:1. Introduction
2. Materials and Methods
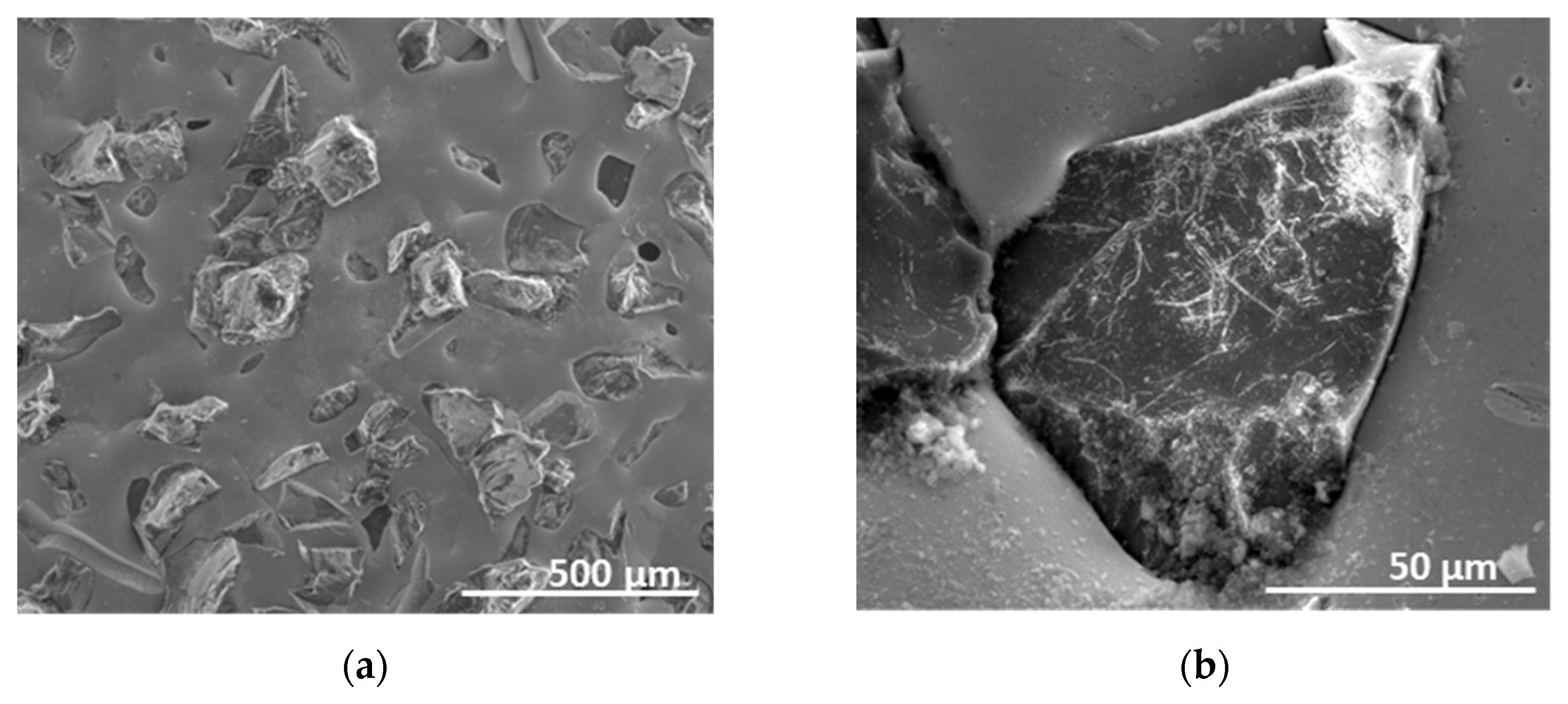
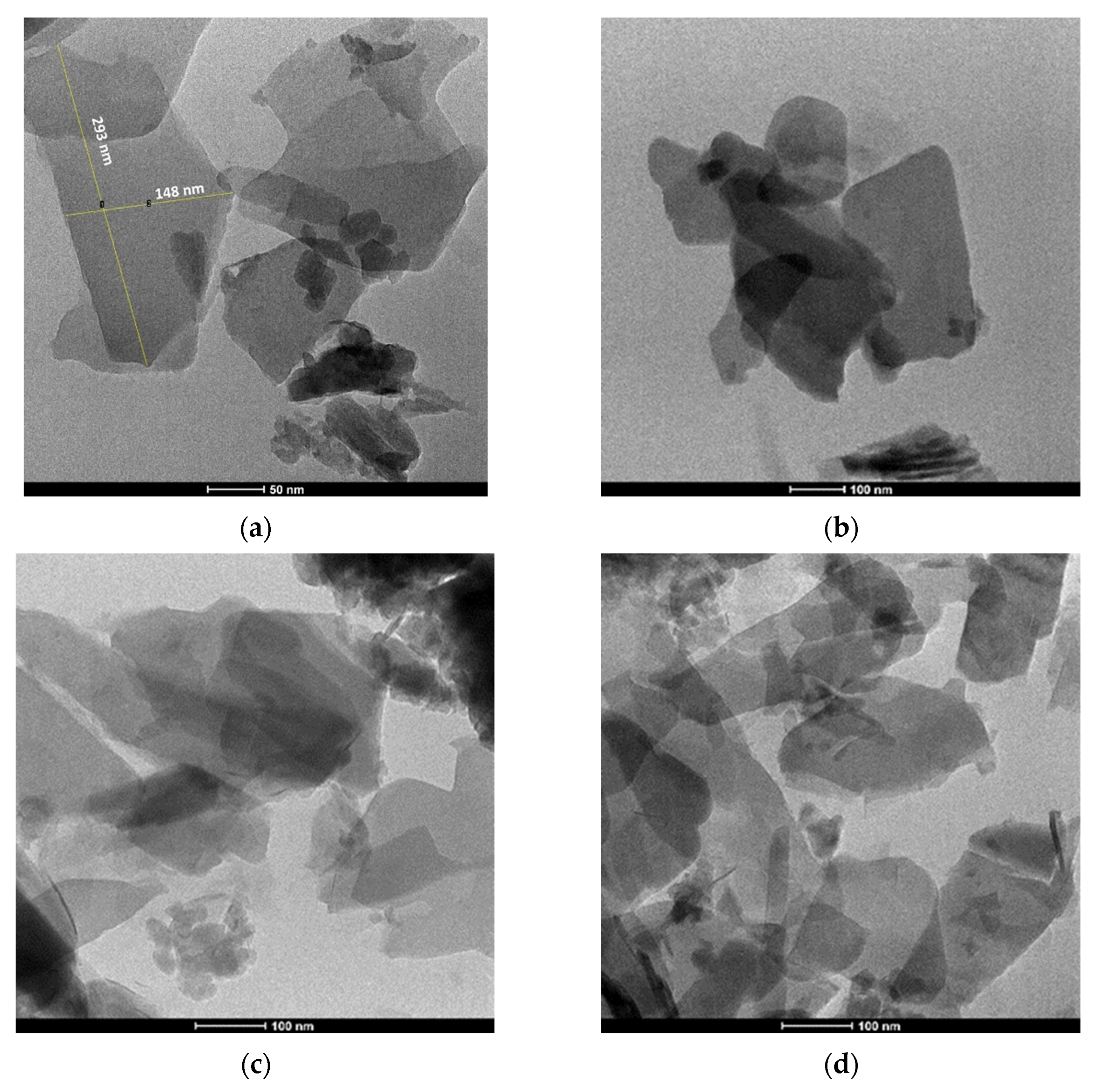
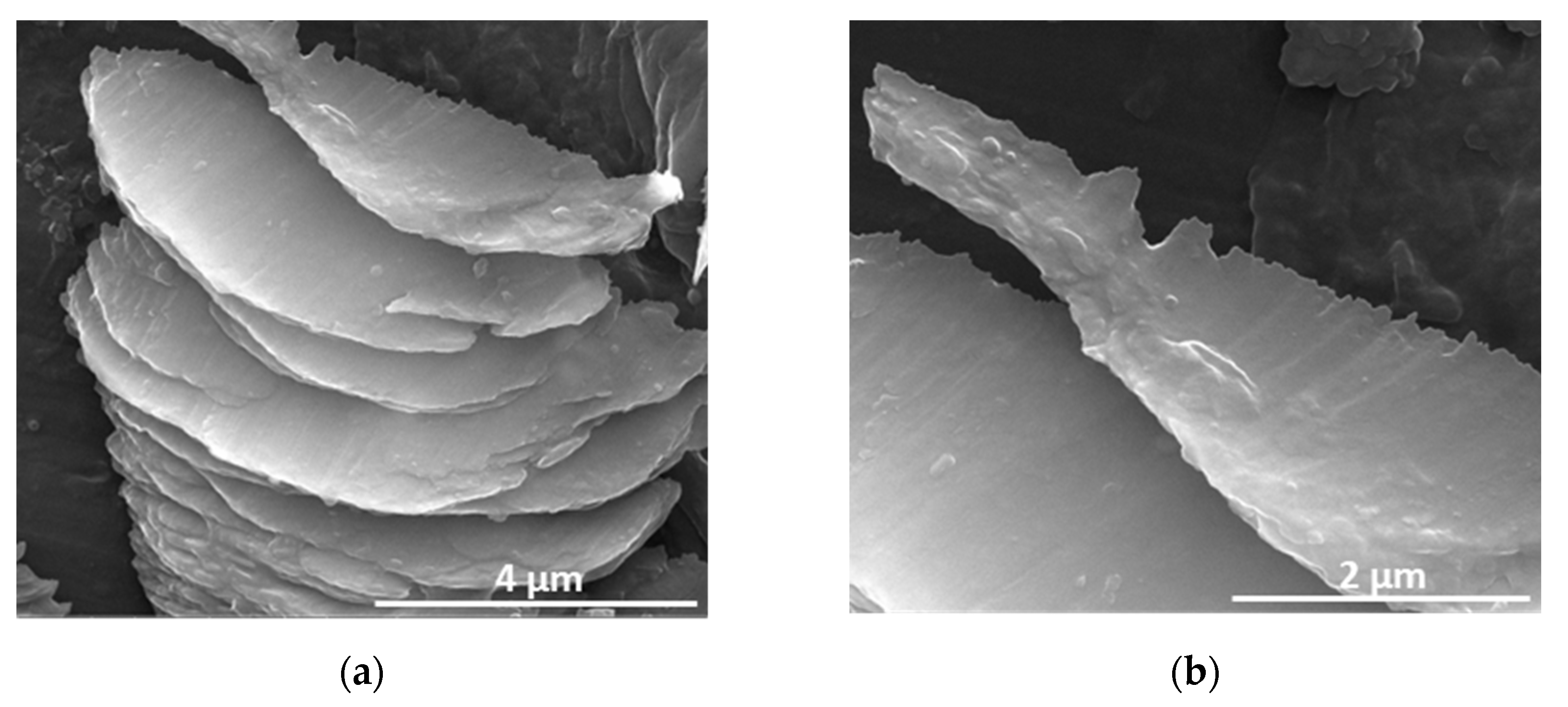
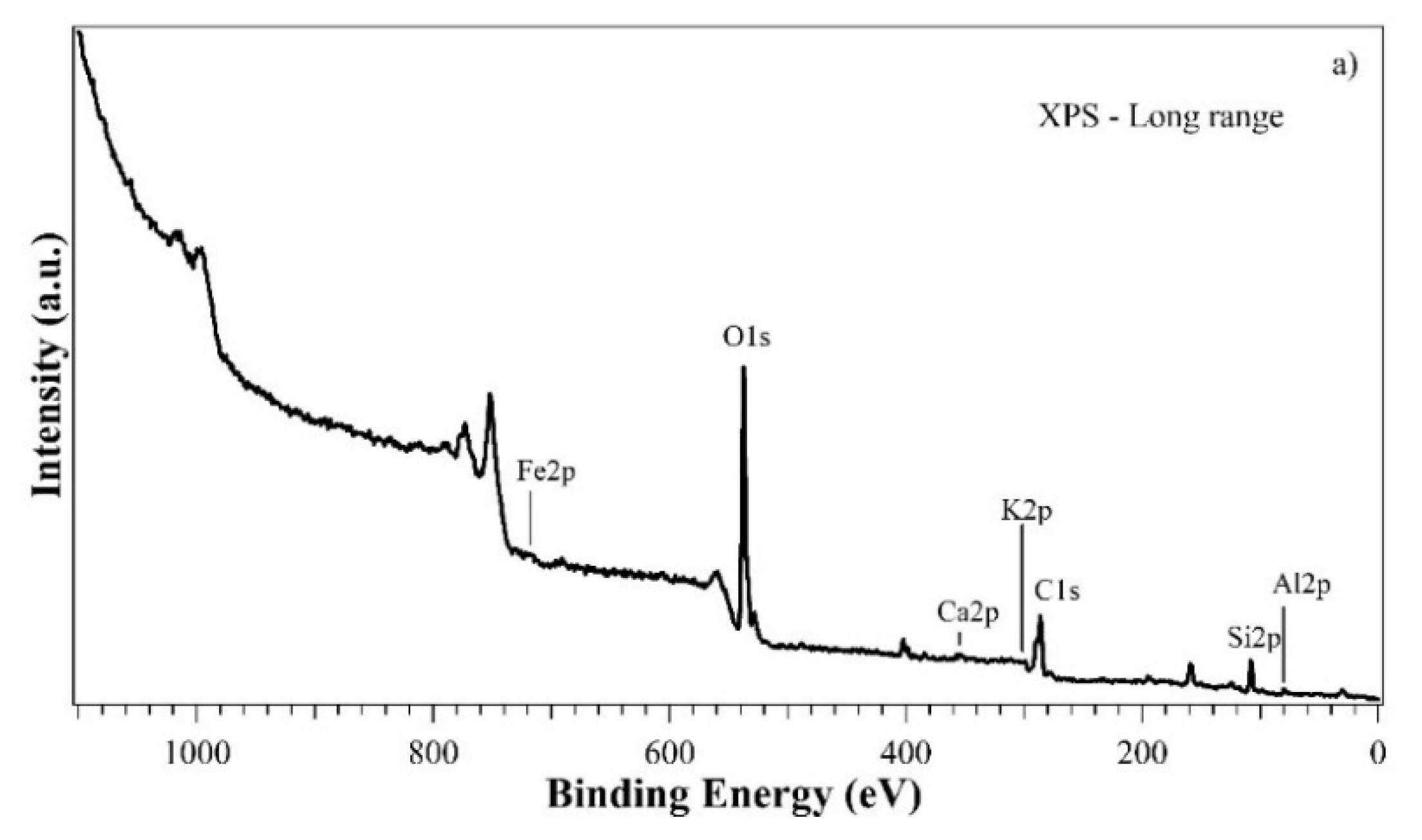
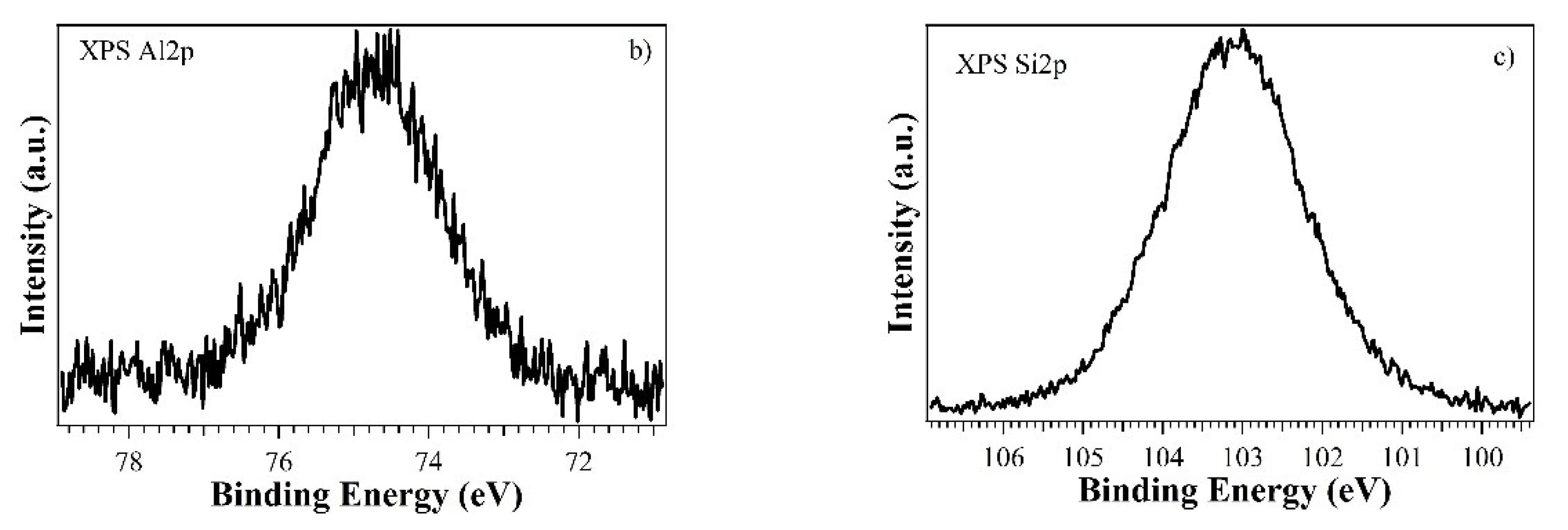
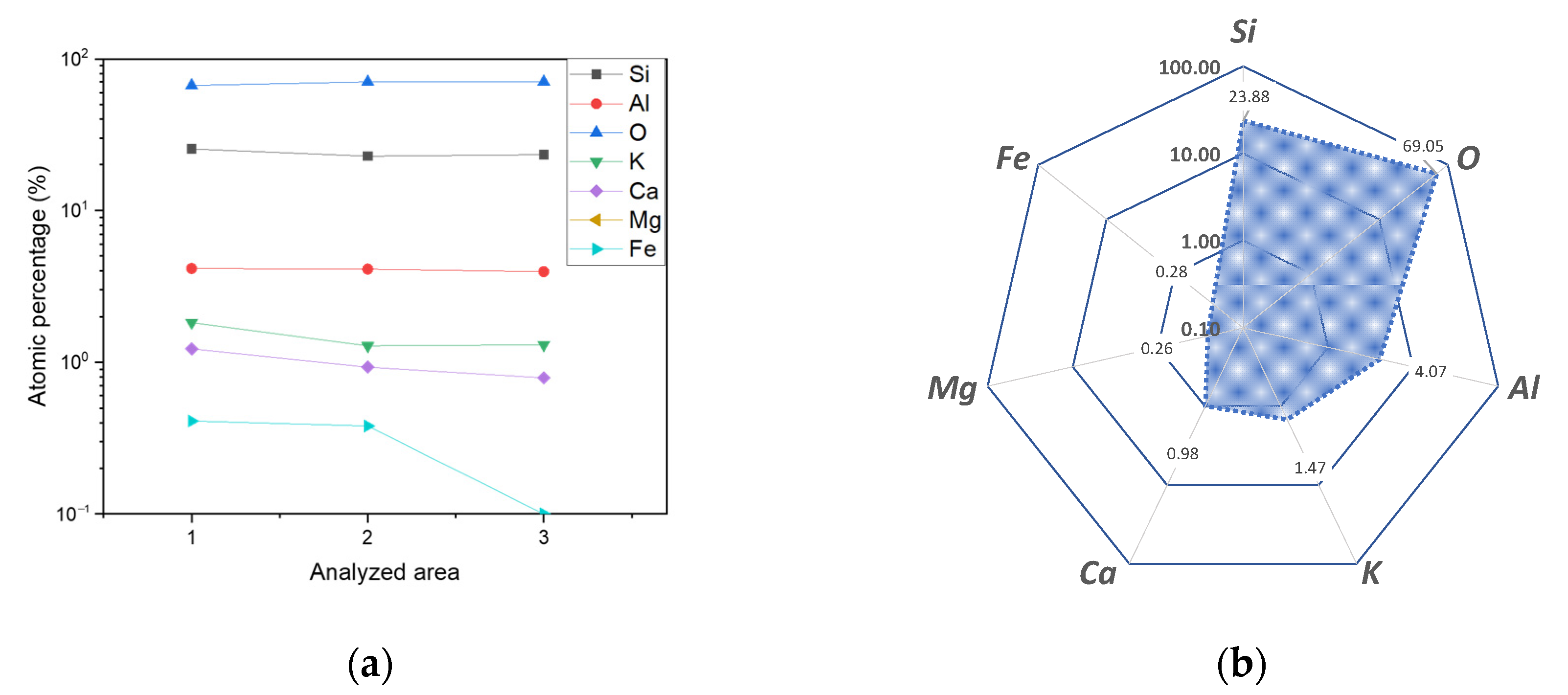
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gargiulo, N.; Shibata, K.; Peluso, A.; Aprea, P.; Valente, T.; Pezzotti, G.; Shiono, T.; Caputo, D. Reinventing rice husk ash: Derived NaX zeolite as a high-performing CO2 adsorbent. Int. J. Environ. Sci. Technol. 2018, 15, 1543–1550. [Google Scholar] [CrossRef]
- Djaeni, M.; Kurniasari, L.; Purbasari, A.; Sasongko, S.B. Activation of natural zeolite as water adsorbent for mixed-adsorption drying. In Proceedings of the 1st International Conference on Materials Engineering (ICME) and 3rd AUN/SEED-Net Regional Conference on Materials (RCM), Yogyakarta, Indonesia, 25–26 November 2010. [Google Scholar]
- Djaeni, M.; Kurniasari, L.; Sasongko, S.B. Preparation of natural zeolite for air dehumidification in food drying. Int. J. Sci. Eng. 2015, 8, 80–83. [Google Scholar]
- Cruz, A.J.; Pires, J.; Carvalho, A.P.; Brotas De Carvalho, M. Adsorption of Acetic Acid by Activated Carbons, Zeolites, and Other Adsorbent Materials Related with the Preventive Conservation of Lead Objects in Museum Showcases. J. Chem. Eng. Data. 2004, 49, 725–731. [Google Scholar] [CrossRef]
- Sippel, K.H.; Quiocho, F.A. Ion-dipole interactions and their functions in proteins. Protein Sci. 2015, 24, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Bol, R.A.; Harkness, D.D. The Use of Zeolite Molecular Sieves for Trapping Low Concentrations of CO2 from Environmental Atmospheres. Radiocarbon 1995, 37, 643–647. [Google Scholar] [CrossRef]
- Li, H.X.; Donohue, J.M.; Cormier, W.E.; Chu, Y.F. Application of zeolites as hydrocarbon traps in automotive emission controls. Stud. Surf. Sci. Catal. 2005, 158, 1375–1382. [Google Scholar]
- Hardie, S.M.L.; Garnett, M.H.; Fallick, A.E.; Rowland, A.P.; Ostle, N.J. Carbon Dioxide Capture Using a Zeolite Molecular Sieve Sampling System for Isotopic Studies (13C and 14C) of Respiration. Radiocarbon 2005, 47, 441–451. [Google Scholar] [CrossRef]
- Mastinu, A.; Kumar, A.; Maccarinelli, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Gianoncelli, A.; Memo, M. Zeolite Clinoptilolite: Therapeutic Virtues of an Ancient Mineral. Molecules 2019, 24, 1517. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, I.I.; Knyazeva, E.E. Micro–mesoporous materials obtained by zeolite recrystallization: Synthesis, characterization and catalytic applications. Chem. Soc. Rev. 2013, 42, 3671–3688. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Sprynskyy, M.; Terzyk, A.P.; Lebedynets, M.; Namieśnik, J.; Buszewski, B. Porous structure of natural and modified clinoptilolites. J. Colloid Interface Sci. 2006, 297, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, Y.; Zhao, H.; Zhang, H.; Ye, Z.; Liu, P.; Zhang, Y.; Tang, Y. One-Pot Exfoliation and Functionalization of Zeolite Nanosheets for Protection of Paper-Based Relics. ACS Appl. Nano Mater. 2021, 4, 10645–10656. [Google Scholar] [CrossRef]
- Gruenert, W.; Muhler, M.; Schroeder, K.P.; Sauer, J.; Schloegl, R. Investigations of Zeolites by Photoelectron and Ion Scattering Spectroscopy. 2. A New Interpretation of XPS Binding Energy Shifts in Zeolites. J. Phys. Chem. 1994, 98, 10920–10929. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiavo, L.; Boccia, V.; Aversa, L.; Verucchi, R.; Carotenuto, G.; Valente, T. Natural Clinoptilolite Nanoplatelets Production by a Friction-Based Technology. Mater. Proc. 2023, 14, 11. https://doi.org/10.3390/IOCN2023-14474
Schiavo L, Boccia V, Aversa L, Verucchi R, Carotenuto G, Valente T. Natural Clinoptilolite Nanoplatelets Production by a Friction-Based Technology. Materials Proceedings. 2023; 14(1):11. https://doi.org/10.3390/IOCN2023-14474
Chicago/Turabian StyleSchiavo, Loredana, Vincenzo Boccia, Lucrezia Aversa, Roberto Verucchi, Gianfranco Carotenuto, and Teodoro Valente. 2023. "Natural Clinoptilolite Nanoplatelets Production by a Friction-Based Technology" Materials Proceedings 14, no. 1: 11. https://doi.org/10.3390/IOCN2023-14474
APA StyleSchiavo, L., Boccia, V., Aversa, L., Verucchi, R., Carotenuto, G., & Valente, T. (2023). Natural Clinoptilolite Nanoplatelets Production by a Friction-Based Technology. Materials Proceedings, 14(1), 11. https://doi.org/10.3390/IOCN2023-14474






